Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium
- PMID: 35746786
- PMCID: PMC9228783
- DOI: 10.3390/v14061315
Investigating COVID-19 Vaccine Impact on the Risk of Hospitalisation through the Analysis of National Surveillance Data Collected in Belgium
Abstract
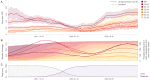
The national vaccination campaign against SARS-CoV-2 started in January 2021 in Belgium. In the present study, we aimed to use national hospitalisation surveillance data to investigate the recent evolution of vaccine impact on the risk of COVID-19 hospitalisation. We analysed aggregated data from 27,608 COVID-19 patients hospitalised between October 2021 and February 2022, stratified by age category and vaccination status. For each period, vaccination status, and age group, we estimated risk ratios (RR) corresponding to the ratio between the probability of being hospitalised following SARS-CoV-2 infection if belonging to the vaccinated population and the same probability if belonging to the unvaccinated population. In October 2021, a relatively high RR was estimated for vaccinated people > 75 years old, possibly reflecting waning immunity within this group, which was vaccinated early in 2021 and invited to receive the booster vaccination at that time. In January 2022, a RR increase was observed in all age categories coinciding with the dominance of the Omicron variant. Despite the absence of control for factors like comorbidities, previous infections, or time since the last administered vaccine, we showed that such real-time aggregated data make it possible to approximate trends in vaccine impact over time.
Keywords: COVID-19; SARS-CoV-2; hospitalisation surveillance; risk ratio; vaccination impact.
Conflict of interest statement
No conflict of interest.
Figures
References
-
- The RECOVERY Collaborative Group. Horby P.W., Mafham M., Peto L., Campbell M., Pessoa-Amorim G., Spata E., Staplin N., Emberson J.R., Prudon B., et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.15.21258542. - DOI
-
- Zuil M., Benítez I.D., Cabo-Gambín R., Manzano Senra C., Moncusí-Moix A., Gort-Paniello C., de Gonzalo-Calvo D., Molinero M., Vengoechea Aragoncillo J.J., Comella T., et al. Clinical management and outcome differences between first and second waves among COVID-19 hospitalized patients: A regional prospective observational cohort. PLoS ONE. 2021;16:e0258918. doi: 10.1371/journal.pone.0258918. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

