Eliciting anti-cancer immunity by genetically engineered multifunctional exosomes
- PMID: 35746867
- PMCID: PMC9481992
- DOI: 10.1016/j.ymthe.2022.06.013
Eliciting anti-cancer immunity by genetically engineered multifunctional exosomes
Abstract
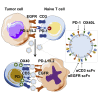
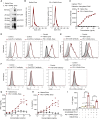
Exosomes are cell-derived nanovesicles involved in regulating intercellular communications. In contrast to conventional nanomedicines, exosomes are characterized by unique advantages for therapeutic development. Despite their major successes in drug delivery, the full potential of exosomes for immunotherapy remains untapped. Herein we designed genetically engineered exosomes featured with surfaced-displayed antibody targeting groups and immunomodulatory proteins. Through genetic fusions with exosomal membrane proteins, Expi293F cell-derived exosomes were armed with monoclonal antibodies specific for human T-cell CD3 and epidermal growth factor receptor (EGFR) as well as immune checkpoint modulators, programmed death 1 (PD-1) and OX40 ligand (OX40L). The resulting genetically engineered multifunctional immune-modulating exosomes (GEMINI-Exos) can not only redirect and activate T cells toward killing EGFR-positive triple negative breast cancer (TNBC) cells but also elicit robust anti-cancer immunity, giving rise to highly potent inhibition against established TNBC tumors in mice. GEMINI-Exos represent candidate agents for immunotherapy and may offer a general strategy for generating exosome-based immunotherapeutics with desired functions and properties.
Keywords: exosomes; extracellular vesicles; immunotherapy; protein engineering; synthetic biology; triple negative breast cancer.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Boulanger C.M., Loyer X., Rautou P.-E., Amabile N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017;14:259–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

