A roadmap to understanding diversity and function of coral reef-associated fungi
- PMID: 35746877
- PMCID: PMC9629503
- DOI: 10.1093/femsre/fuac028
A roadmap to understanding diversity and function of coral reef-associated fungi
Abstract
Tropical coral reefs are hotspots of marine productivity, owing to the association of reef-building corals with endosymbiotic algae and metabolically diverse bacterial communities. However, the functional importance of fungi, well-known for their contribution to shaping terrestrial ecosystems and global nutrient cycles, remains underexplored on coral reefs. We here conceptualize how fungal functional traits may have facilitated the spread, diversification, and ecological adaptation of marine fungi on coral reefs. We propose that functions of reef-associated fungi may be diverse and go beyond their hitherto described roles of pathogens and bioeroders, including but not limited to reef-scale biogeochemical cycles and the structuring of coral-associated and environmental microbiomes via chemical mediation. Recent technological and conceptual advances will allow the elucidation of the physiological, ecological, and chemical contributions of understudied marine fungi to coral holobiont and reef ecosystem functioning and health and may help provide an outlook for reef management actions.
Keywords: chemical mediation; ecosystem functioning; interkingdom interactions; marine fungi; nutrient cycling; probiotics.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures

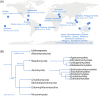



References
-
- Agrawal S, Adholeya A, Barrow CJet al. Marine fungi: an untapped bioresource for future cosmeceuticals. Phytochem Lett. 2018;23:15–20.
-
- Ainsworth TDD, Fordyce AJJ, Camp EFF.. The other microeukaryotes of the coral reef microbiome. Trends Microbiol. 2017;25:980–91. - PubMed
-
- Alain K, Querellou J.. Cultivating the uncultured: limits, advances and future challenges. Extremophiles. 2009;13:583–94. - PubMed

