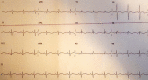
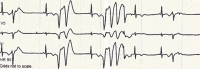
Loperamide-induced ventricular tachycardia storm
- PMID: 35747063
- PMCID: PMC9063705
- DOI: 10.5837/bjc.2021.046
Loperamide-induced ventricular tachycardia storm
Abstract
Loperamide is an over-the-counter, peripherally-acting, μ-opioid receptor agonist commonly used in the treatment of diarrhoea. It has increasingly been recognised as a potential drug of misuse, having previously been thought to have low potential for abuse owing to its low bioavailability and poor penetration of the central nervous system. High doses can result in life-threatening cardiac-toxicity. We present a case of a young woman who had been self-treating her depression with high doses of loperamide for one year, who then presented to hospital with syncope secondary to ventricular tachycardia (VT). While in the emergency department (ED) the patient had monomorphic pulseless VT requiring electrical cardioversion multiple times. Transfer to a tertiary cardiac centre was immediately arranged after she was stabilised and intubated. This complicated the diagnostic process as a thorough history could not be obtained on arrival to the tertiary centre, which meant the loperamide misuse only came to light multiple days into admission, after the patient was extubated. The final diagnosis of loperamide-induced secondary long-QT syndrome was made and the patient made a full recovery.
Keywords: loperamide; ventricular tachycardia.
Copyright © 2021 Medinews (Cardiology) Limited.
Conflict of interest statement
Conflicts of interest None declared.
Figures
References
-
- Food and Drugs Administration FDA warns about serious heart problems with high doses of the antidiarrheal medicine loperamide (Imodium), including from abuse and misuse. [accessed 6 July 2016];Safety Announcement. Available at: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fdadrug-safety-podcas....
-
- Parker BM, Rao T, Matta A, et al. Loperamide induced cardiac arrhythmia successfully supported with veno-arterial ECMO (VA-ECMO), molecular adsorbent recirculating system (MARS) and continuous renal replacement therapy (CRRT) Clin Toxicol. 2019;57:1118–1122. doi: 10.1080/15563650.2019.1580370. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials