Network meta-analysis of immune-oncology monotherapy as first-line treatment for advanced non-small-cell lung cancer in patients with PD-L1 expression ⩾50
- PMID: 35747163
- PMCID: PMC9210099
- DOI: 10.1177/17588359221105024
Network meta-analysis of immune-oncology monotherapy as first-line treatment for advanced non-small-cell lung cancer in patients with PD-L1 expression ⩾50
Erratum in
-
Corrigendum to "Network meta-analysis of immune-oncology monotherapy as first-line treatment for advanced non-small-cell lung cancer in patients with PD-L1 expression ⩾50%".Ther Adv Med Oncol. 2022 Sep 13;14:17588359221127078. doi: 10.1177/17588359221127078. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 36119642 Free PMC article.
Abstract
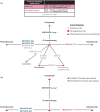
Background: For patients with advanced non-small-cell lung cancer (NSCLC) and high (⩾50%) programmed cell death-ligand 1 (PD-L1) expression, effective first-line immune-oncology monotherapies with significant survival benefits are approved, cemiplimab being the most recent. In a phase III trial, cemiplimab demonstrated significantly improved overall survival (OS) and progression-free survival (PFS) versus chemotherapy in patients with advanced NSCLC and PD-L1 ⩾50%. A systematic literature review and network meta-analysis (NMA) was conducted to identify/compare the efficacy/safety of cemiplimab versus pembrolizumab or other immune-oncology monotherapies from randomized-controlled trials (RCTs) published in November 2010-2020.
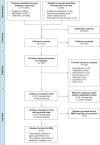
Methods: Relevant RCTs were identified by searching databases and conference proceedings as per ISPOR, NICE, and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. NMA with time-varying hazard ratios (HRs) was performed for OS and PFS. Analyses were conducted for objective response rate (ORR) and safety/tolerability. Fixed-effect models were used due to limited evidence. Various sensitivity analyses were conducted to validate the base case analyses.
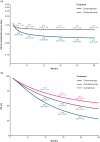
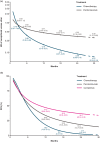
Results: The feasibility assessment determined that EMPOWER-Lung 1, KEYNOTE-024, and KEYNOTE-042 trials were eligible. IMpower110 was excluded because an incompatible PD-L1 assay (SP142) was used for patient selection. For first-line advanced NSCLC with PD-L1 ⩾50%, cemiplimab was associated with statistically significant improvements in PFS [HR (95% credible interval [CrI]): 0.65 (0.50-0.86), 1-12 months] and ORR [odds ratio (OR) (95% CrI): 1.64 (1.04-2.62)], and comparable OS [HR (95% CrI): 0.77 (0.54-1.10), 1-12 months] versus pembrolizumab. There was no evidence of differences between cemiplimab and pembrolizumab for Grade 3-5 adverse events (AEs) [OR (95% CrI): 1.47 (0.83-2.60)], immune-mediated AEs [1.75 (0.33-7.49)], and all-cause discontinuation due to AEs [1.21 (0.58-2.61)].
Conclusions: Considering the limitations of indirect treatment comparisons, in patients with advanced NSCLC and PD-L1 ⩾50%, cemiplimab monotherapy demonstrated significant improvements in PFS and ORR, comparable OS, and no evidence of differences in safety/tolerability versus pembrolizumab.
Keywords: IO monotherapy; PD-L1 expression; cemiplimab; cemiplimab monotherapy; network meta-analysis; non-small-cell lung cancer; systematic literature review.
© The Author(s), 2022.
Conflict of interest statement
Conflict of interest statement: Nick Freemantle declares consulting services for ALK, Allergan, Aimmune, AstraZeneca, Gilead, Grifols, Ipsen, MSD, Novartis, Novo Nordisk, Sanofi Aventis, and Vertex; and speaking services for Abbott Singapore and Sanofi Aventis. Yingxin Xu, Chieh-I Chen, Andreas Kuznik, Jean-Francois Pouliot, Giuseppe Gullo, and Petra Rietschel are employees and stockholders of Regeneron Pharmaceuticals, Inc. Florence R. Wilson, Sam Keeping, Keith Chan, and Emily Glowienka are employees of Precision HEOR and received funding to produce this work. Patricia Guyot, Gerasimos Konidaris, and Kokuvi Atsou are employees of Sanofi and may own shares or stock options in the company.
Figures
Similar articles
-
Comparison of the profiles of first-line PD-1/PD-L1 inhibitors for advanced NSCLC lacking driver gene mutations: a systematic review and Bayesian network meta-analysis.Ther Adv Chronic Dis. 2023 Oct 11;14:20406223231189224. doi: 10.1177/20406223231189224. eCollection 2023. Ther Adv Chronic Dis. 2023. PMID: 37841212 Free PMC article.
-
Comparative Efficacy and Safety of Anti-PD-1/PD-L1 for the Treatment of Non-Small Cell Lung Cancer: A Network Meta-Analysis of 13 Randomized Controlled Studies.Front Oncol. 2022 May 10;12:827050. doi: 10.3389/fonc.2022.827050. eCollection 2022. Front Oncol. 2022. PMID: 35619899 Free PMC article.
-
Comparative long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer: a systematic review and network meta-analysis.Front Immunol. 2024 Jul 11;15:1375136. doi: 10.3389/fimmu.2024.1375136. eCollection 2024. Front Immunol. 2024. PMID: 39072325 Free PMC article.
-
Comparative outcomes of first-line PD-1/PD-L1 inhibitors plus chemotherapy for advanced squamous non-small cell lung cancer: a systematic review and network meta-analysis of randomized clinical trials.Transl Lung Cancer Res. 2025 Feb 28;14(2):563-574. doi: 10.21037/tlcr-2025-83. Epub 2025 Feb 27. Transl Lung Cancer Res. 2025. PMID: 40114962 Free PMC article.
-
A Network Meta-Analysis of Cancer Immunotherapies Versus Chemotherapy for First-Line Treatment of Patients With Non-Small Cell Lung Cancer and High Programmed Death-Ligand 1 Expression.Front Oncol. 2021 Jul 9;11:676732. doi: 10.3389/fonc.2021.676732. eCollection 2021. Front Oncol. 2021. PMID: 34307144 Free PMC article. Review.
Cited by
-
Impact of the treatment crossover design on comparative efficacy in EMPOWER-Lung 1: Cemiplimab monotherapy as first-line treatment of advanced non-small cell lung cancer.Front Oncol. 2023 Apr 4;12:1081729. doi: 10.3389/fonc.2022.1081729. eCollection 2022. Front Oncol. 2023. PMID: 37082098 Free PMC article.
-
First-line immunotherapy efficacy in advanced squamous non-small cell lung cancer with PD-L1 expression ≥50%: a network meta-analysis of randomized controlled trials.Front Oncol. 2024 Apr 24;14:1365255. doi: 10.3389/fonc.2024.1365255. eCollection 2024. Front Oncol. 2024. PMID: 38725635 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. - PubMed
-
- American Cancer Society. Cancer facts and figures, 2018, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-... (accessed 6 June 2022).
-
- Cancer.net. Lung cancer - non-small cell: statistics, https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (2020, accessed 6 June 2022).
LinkOut - more resources
Full Text Sources
Research Materials

