Effect of early two-dose measles vaccination on childhood mortality and modification by maternal measles antibody in Guinea-Bissau, West Africa: A single-centre open-label randomised controlled trial
- PMID: 35747181
- PMCID: PMC9156892
- DOI: 10.1016/j.eclinm.2022.101467
Effect of early two-dose measles vaccination on childhood mortality and modification by maternal measles antibody in Guinea-Bissau, West Africa: A single-centre open-label randomised controlled trial
Abstract
Background: Early 2-dose measles vaccine (MV) at 4 and 9 months of age vs. the WHO strategy of MV at 9 months of age reduced all-cause child mortality in a previous trial. We aimed to test two hypotheses: 1) a 2-dose strategy reduces child mortality between 4 and 60 months of age by 30%; 2) receiving early MV at 4 months in the presence versus absence of maternal measles antibodies (MatAb) reduces child mortality by 35%.
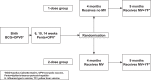
Methods: Single-centre open-label community-based randomised controlled trial in Guinea-Bissau, with 2:1 block-randomisation by sex to a 2-dose (4 + 9 months) vs. 1-dose (9 months) MV strategy. Healthy children were eligible 4 weeks after the 3rd diphtheria-tetanus-pertussis-containing vaccine. Before randomisation a blood sample was collected to determine MatAb level. The primary outcome was all-cause mortality. Hazard ratios (HR) were derived from Cox regression in the per protocol population. We tested for interactions with national campaigns with oral polio vaccine (C-OPV). Trial registration: NCT01486355.
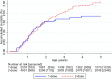
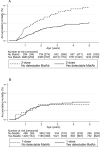
Findings: Between August 2011-April 17th 2015, 6,636 children were enroled, 6,598[n2-dose=4,397; n1-dose=2,201] were included in the analysis of the primary outcome, The HR(2-dose/1-dose) between 4 and 60 months was 1.38 (95%CI: 0.92-2.06) [deaths: n2-dose=90; n1-dose=33]. Before the 9-month MV and the HR(1-dose/no dose) was 0.94 (0.45-1.96) [deaths: n2-dose=21; n1-dose=11]. The HR(2-dose/1-dose) was 0.81 (0.29-2.22) for children, who received no C-OPV [deaths/children: n2-dose=10/2,801; n1-dose=6/1,365], and 4.73 (1.44-15.6) for children, who received C-OPV before and after enrolment (p for interaction=0.027) [deaths/children: n2-dose=27/1,602; n1-dose=3/837]. In the 2-dose group receiving early MV at 4 months, mortality was 50% (20-68%) lower for those vaccinated in the presence of MatAb vs. the absence of MatAb [deaths/children: nMatAb=51/3,132; nnoMatAb=31/1,028].
Interpretation: The main result contrasts with previous findings but may, though based on a small number of events, be explained by frequent OPV campaigns that reduced the mortality rate, but apparently interacted negatively with early MV. The beneficial non-specific effects of MV in the presence of MatAb should be investigated further.
Funding: ERC, Danish National Research Foundation, the Danish Council for Development Research, Ministry of Foreign Affairs, Novo Nordisk Foundation, European Union and the Lundbeck Foundation.
Keywords: Heterologous effecs; Maternal antibody; Maternal priming; Measles; Mortality; Non-specific effects; Vaccines.
© 2022 The Authors.
Conflict of interest statement
OB received a scholarship from the Lundbeck Foundation. All other authors declare no competing interests.
Figures

Similar articles
-
The effect of early measles vaccination on morbidity and growth: A randomised trial from Guinea-Bissau.Vaccine. 2020 Mar 4;38(11):2487-2494. doi: 10.1016/j.vaccine.2020.01.096. Epub 2020 Feb 13. Vaccine. 2020. PMID: 32061387 Clinical Trial.
-
Does oral polio vaccine have non-specific effects on all-cause mortality? Natural experiments within a randomised controlled trial of early measles vaccine.BMJ Open. 2016 Dec 23;6(12):e013335. doi: 10.1136/bmjopen-2016-013335. BMJ Open. 2016. PMID: 28011813 Free PMC article. Clinical Trial.
-
Research protocol of two concurrent cluster-randomized trials: Real-life Effect of a CAMPaign with Measles Vaccination (RECAMP-MV) and Real-life Effect of a CAMPaign with Oral Polio Vaccination (RECAMP-OPV) on mortality and morbidity among children in rural Guinea-Bissau.BMC Public Health. 2019 Nov 11;19(1):1506. doi: 10.1186/s12889-019-7813-y. BMC Public Health. 2019. PMID: 31711464 Free PMC article.
-
Measles vaccination and reduced child mortality: Prevention of immune amnesia or beneficial non-specific effects of measles vaccine?J Infect. 2023 Oct;87(4):295-304. doi: 10.1016/j.jinf.2023.07.010. Epub 2023 Jul 22. J Infect. 2023. PMID: 37482223 Review.
-
Revaccination with Live Attenuated Vaccines Confer Additional Beneficial Nonspecific Effects on Overall Survival: A Review.EBioMedicine. 2016 Aug;10:312-7. doi: 10.1016/j.ebiom.2016.07.016. Epub 2016 Jul 15. EBioMedicine. 2016. PMID: 27498365 Free PMC article. Review.
Cited by
-
Measles, mumps, and rubella vaccine at age 6 months and hospitalisation for infection before age 12 months: randomised controlled trial.BMJ. 2023 Jun 7;381:e072724. doi: 10.1136/bmj-2022-072724. BMJ. 2023. PMID: 37286215 Free PMC article. Clinical Trial.
-
Measles vaccines and non-specific effects on mortality or morbidity: A systematic review and meta-analysis.PLoS One. 2025 Jul 2;20(7):e0321982. doi: 10.1371/journal.pone.0321982. eCollection 2025. PLoS One. 2025. PMID: 40601595 Free PMC article.
-
The mortality effects of disregarding the strategy to save doses of measles vaccine: a cluster-randomised trial in Guinea-Bissau.BMJ Glob Health. 2021 May;6(5):e004328. doi: 10.1136/bmjgh-2020-004328. BMJ Glob Health. 2021. PMID: 33941513 Free PMC article. Clinical Trial.
-
Effects of Neonatal BCG-Japan Versus BCG-Russia Vaccination on Overall Mortality and Morbidity: Randomized Controlled Trial From Guinea-Bissau (BCGSTRAIN II).Open Forum Infect Dis. 2024 Feb 1;11(3):ofae057. doi: 10.1093/ofid/ofae057. eCollection 2024 Mar. Open Forum Infect Dis. 2024. PMID: 38500576 Free PMC article. Clinical Trial.
-
Improving retrospective data on recent household deaths: a multi-arm randomized trial in Guinea-Bissau.Int J Epidemiol. 2025 Feb 16;54(2):dyaf009. doi: 10.1093/ije/dyaf009. Int J Epidemiol. 2025. PMID: 39993265 Free PMC article. Clinical Trial.
References
-
- The Kasongo Project Team Influence of measles vaccination on survival pattern of 7-35-month-old children in Kasongo, Zaire. Lancet. 1981;317(8223):764–767. - PubMed
-
- Aaby P., Bukh J., Lisse I.M., Smits A.J. Measles vaccination and reduction in child mortality: a community study from GuineaBissau. J Infect. 1984;8:1321. - PubMed
-
- Aaby P., Bhuyia A., Nahar L., Knudsen K., de Francisco A., Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol. 2003;32:106–115. - PubMed
-
- Aaby P., Garly M.L., Balé C., et al. Survival of previously measles-vaccinated and measles-unvaccinated children in an emergency situation: an unplanned study. Pediatr Infect Dis J. 2003;22:798–803. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

