Altered Reward Processing and Sex Differences in Chronic Pain
- PMID: 35747210
- PMCID: PMC9211769
- DOI: 10.3389/fnins.2022.889849
Altered Reward Processing and Sex Differences in Chronic Pain
Abstract
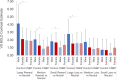
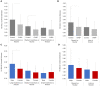
Chronic pain and reward processing are understood to be reciprocally related to one another. Previous studies of reward processing in chronic pain patients have reported incongruent findings. While several factors likely contribute to these disparate findings, these previous studies did not stratify their analyses by sex-a factor previously shown to robustly impact reward-related responses. Thus, we examined sex as a factor of interest in level of striatal activation during anticipation of monetary incentives among patients with chronic non-specific back pain and healthy controls (HC). This study utilized functional magnetic resonance imaging during a monetary incentive delay task to evaluate reward and loss responsivity in the striatum among males and females with and without chronic pain (N = 90). Group, sex, and group-by-sex interactions were analyzed via repeated measures analysis of variance. Among HC, males exhibited significantly greater blood oxygen level dependent (BOLD) signal in the striatum during reward anticipation, particularly during large reward trials. By contrast, no significant sex differences were observed among patients. A significant group-by-sex interaction was also observed, revealing diminished BOLD responses among males with chronic pain relative to control males. These results provide novel evidence of sex-specific reductions in anticipatory responses to reward in patients with chronic pain. Altered striatal reward responsivity among males, but not females, suggests that the reward systems of males and females are uniquely disrupted by chronic pain, and highlights the value of including sex as a factor of interest in future studies of reward responsivity in the context of persistent pain.
Keywords: chronic pain; fMRI; reward processing; sex differences; striatum.
Copyright © 2022 Baker, Ericksen, Koppelmans, Mickey, Martucci, Zubieta and Love.
Conflict of interest statement
BM has received research funding from LivaNova and Novartis and consulting fees from Alkermes, and he has served on the advisory board of FutraMed (unpaid). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

