The SAFE Pilot Trial-SAlvage Focal Irreversible Electroporation-For Recurrent Localized Prostate Cancer: Rationale and Study Protocol
- PMID: 35747441
- PMCID: PMC9209638
- DOI: 10.3389/fsurg.2022.900528
The SAFE Pilot Trial-SAlvage Focal Irreversible Electroporation-For Recurrent Localized Prostate Cancer: Rationale and Study Protocol
Abstract
Introduction: Currently, the majority of prostate cancer (PCa) recurrences after non-surgical first-line treatment are managed with androgen-deprivation therapy (ADT). Salvage radical prostatectomy (sRP) is a curative alternative to ADT but yields significant morbidity. Preliminary evidence from focal salvage treatments shows similar oncological control but lower morbidity compared to sRP. Among available ablative focal energies, irreversible electroporation (IRE) is a treatment modality that proved promising, especially in treating apical lesions, where PCa most often recurs. Our aim is to test the safety of salvage IRE for recurrent PCa.
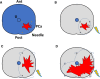
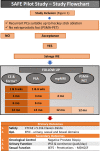
Methods: We performed a single-arm pilot feasibility study (IDEAL stage 2a): SAFE, SAlvage Focal irreversible Electroporation for recurrent localized PCa. Twenty patients with biopsy-proven PCa recurrence after primary non-surgical (radiation or ablation) treatment were included. All men will undergo mpMRI ± targeted biopsies, pre-operative PSMA-PET staging before inclusion and sIRE. Outcomes will be evaluated through internationally validated questionnaires and morbidity scales. All men will undergo a control biopsy at one year.
Results: Primary objectives were the evaluation of the safety of sIRE (and patients' quality of life) after treatment. Secondary objectives were the evaluation of functional outcomes, namely, continence and erectile function changes and evaluation of short-term oncological efficacy.
Conclusions: SAFE is the second pilot study to evaluate sIRE and the first one performed according to the most recent diagnostic and staging imaging standards. sIRE may provide a curative option for recurrent PCa together with lower comorbidities compared to sRP.
Keywords: PSMA-PET/CT; biochemical recurrence (BCR); focal treatment; irreversible electroporation (IRE); prostate cancer.
Copyright © 2022 Marra, Shah, D'Agate, Marquis, Calleris, Lunelli, Filippini, Oderda, Gatti, Valerio, Sanchez-Salas, Bossi, Gomez Rivas, Conte, Deandreis, Cussenot, Ricardi and Gontero.
Conflict of interest statement
Luca Lunelli and Olivier Cussenot have worked as consultants and proctors for Angiodynamics, Latham, NY. The publication costs of this study protocol are covered by Angiodynamics, Latham, NY.
Figures

References
-
- Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomized study. Lancet Oncol. (2010) 11:1066–73. 10.1016/S1470-2045(10)70223-0 - DOI - PubMed
-
- Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomized, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. (2016) 17:1047–60. 10.1016/S1470-2045(16)30102-4 - DOI - PMC - PubMed
-
- Michalski JM, Moughan J, Purdy J, Bosch W, Bruner DW, Bahary JP, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. (2018) 4:e180039. 10.1001/JAMAONCOL.2018.0039 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

