Enhancing accreditation outcomes for medical laboratories on the Strengthening Laboratory Management Toward Accreditation programme in Kenya via a rapid results initiative
- PMID: 35747559
- PMCID: PMC9210179
- DOI: 10.4102/ajlm.v11i1.1614
Enhancing accreditation outcomes for medical laboratories on the Strengthening Laboratory Management Toward Accreditation programme in Kenya via a rapid results initiative
Abstract
Background: Since 2010, Kenya has used SLIPTA to prepare and improve quality management systems in medical laboratories to achieve ISO 15189 accreditation. However, less than 10% of enrolled laboratories had done so in the initial seven years of SLMTA implementation.
Objective: We described Kenya's experience in accelerating medical laboratories on SLMTA to attain ISO 15189 accreditation.
Methods: From March 2017 to July 2017, an aggressive top-down approach through high-level management stakeholder engagement for buy-in, needs-based expedited SLIPTA mentorship and on-site support as a rapid results initiative (RRI) was implemented in 39 laboratories whose quality improvement process had stagnated for 2-7 years. In July 2017, SLIPTA baseline and exit audit average scores on quality essential elements were compared to assess performance.
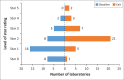
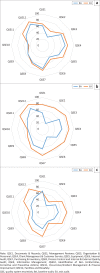
Results: After RRI, laboratories achieving greater than a 2-star SLMTA rating increased significantly from 15 (38%) at baseline to 33 (85%) (p < 0.001). Overall, 34/39 (87%) laboratories received ISO 15189 accreditation within two years of RRI, leading to a 330% increase in the number of accredited laboratories in Kenya. The most improved of the 12 quality system essentials were Equipment Management (mean increase 95% CI: 5.31 ± 1.89) and Facilities and Biosafety (mean increase [95% CI: 4.05 ± 1.78]) (both: p < 0.0001). Information Management and Corrective Action Management remained the most challenging to improve, despite RRI interventions.
Conclusion: High-level advocacy and targeted mentorship through RRI dramatically improved laboratory accreditation in Kenya. Similar approaches of strengthening SLIPTA implementation could improve SLMTA outcomes in other countries with similar challenges.
Keywords: SLIPTA; SLMTA; accreditation; e-SLIPTA checklist; quality systems essentials; rapid results initiative.
© 2022. The Authors.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures
References
-
- SLMTA . Strengthening laboratory management towards accreditation [homepage on the Internet]. [cited 2021 Sep 15]. Available from: https://slmta.org/
LinkOut - more resources
Full Text Sources
