Dual Effects of Presynaptic Membrane Mimetics on α-Synuclein Amyloid Aggregation
- PMID: 35747692
- PMCID: PMC9209734
- DOI: 10.3389/fcell.2022.707417
Dual Effects of Presynaptic Membrane Mimetics on α-Synuclein Amyloid Aggregation
Abstract
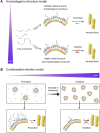
Aggregation of intrinsically disordered α-synuclein (αSN) under various conditions is closely related to synucleinopathies. Although various biological membranes have shown to alter the structure and aggregation propensity of αSN, a thorough understanding of the molecular and mechanical mechanism of amyloidogenesis in membranes remains unanswered. Herein, we examined the structural changes, binding properties, and amyloidogenicity of three variations of αSN mutants under two types of liposomes, 1,2-Dioleoyl-sn-glycero-3-Phosphocholine (DOPC) and presynaptic vesicle mimetic (Mimic) membranes. While neutrally charged DOPC membranes elicited marginal changes in the structure and amyloid fibrillation of αSNs, negatively charged Mimic membranes induced dramatic helical folding and biphasic amyloid generation. At low concentration of Mimic membranes, the amyloid fibrillation of αSNs was promoted in a dose-dependent manner. However, further increases in the concentration constrained the fibrillation process. These results suggest the dual effect of Mimic membranes on regulating the amyloidogenesis of αSN, which is rationalized by the amyloidogenic structure of αSN and condensation-dilution of local αSN concentration. Finally, we propose physicochemical properties of αSN and membrane surfaces, and their propensity to drive electrostatic interactions as decisive factors of amyloidogenesis.
Keywords: Parkinson’s disease; amyloid fibril; electrostatic interaction; helical structure; intermolecular interaction; membrane mimetic; presynaptic vesicle; α-Synuclein.
Copyright © 2022 Lin, Ito, Yoo, Lim, Yu, Kawata and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Membrane-induced initial structure of α-synuclein control its amyloidogenesis on model membranes.Biochim Biophys Acta Biomembr. 2018 Mar;1860(3):757-766. doi: 10.1016/j.bbamem.2017.12.011. Epub 2017 Dec 19. Biochim Biophys Acta Biomembr. 2018. PMID: 29273335
-
An amphiphilic material arginine-arginine-bile acid promotes α-synuclein amyloid formation.Nanoscale. 2023 Jun 1;15(21):9315-9328. doi: 10.1039/d3nr01468a. Nanoscale. 2023. PMID: 37158478
-
Evaluation of membrane models and their composition for islet amyloid polypeptide-membrane aggregation.Biochim Biophys Acta. 2013 Sep;1828(9):2091-8. doi: 10.1016/j.bbamem.2013.05.014. Epub 2013 May 23. Biochim Biophys Acta. 2013. PMID: 23707907
-
Impact of membrane curvature on amyloid aggregation.Biochim Biophys Acta Biomembr. 2018 Sep;1860(9):1741-1764. doi: 10.1016/j.bbamem.2018.04.012. Epub 2018 Apr 28. Biochim Biophys Acta Biomembr. 2018. PMID: 29709613 Free PMC article. Review.
-
Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease.Neuropharmacology. 2003 Jul;45(1):14-44. doi: 10.1016/s0028-3908(03)00140-0. Neuropharmacology. 2003. PMID: 12814657 Review.
Cited by
-
The Role of α-Synuclein in Etiology of Neurodegenerative Diseases.Int J Mol Sci. 2024 Aug 24;25(17):9197. doi: 10.3390/ijms25179197. Int J Mol Sci. 2024. PMID: 39273146 Free PMC article. Review.
-
BeStSel: analysis site for protein CD spectra-2025 update.Nucleic Acids Res. 2025 Jul 7;53(W1):W73-W83. doi: 10.1093/nar/gkaf378. Nucleic Acids Res. 2025. PMID: 40357643 Free PMC article.
-
Graphene Quantum Dots Attenuate TDP-43 Proteinopathy in Amyotrophic Lateral Sclerosis.ACS Nano. 2025 Mar 11;19(9):8692-8710. doi: 10.1021/acsnano.4c15283. Epub 2025 Feb 4. ACS Nano. 2025. PMID: 39901566 Free PMC article.
-
Interactions with tau's microtubule-binding repeats modulate amyloid-β aggregation and toxicity.Nat Chem Biol. 2025 Aug 22. doi: 10.1038/s41589-025-01987-0. Online ahead of print. Nat Chem Biol. 2025. PMID: 40846995
-
Polymorphism in alpha-synuclein oligomers and its implications in toxicity under disease conditions.Front Mol Biosci. 2022 Aug 12;9:959425. doi: 10.3389/fmolb.2022.959425. eCollection 2022. Front Mol Biosci. 2022. PMID: 36032665 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

