Development, Validation, and Comparison of Image-Based, Clinical Feature-Based and Fusion Artificial Intelligence Diagnostic Models in Differentiating Benign and Malignant Pulmonary Ground-Glass Nodules
- PMID: 35747810
- PMCID: PMC9209648
- DOI: 10.3389/fonc.2022.892890
Development, Validation, and Comparison of Image-Based, Clinical Feature-Based and Fusion Artificial Intelligence Diagnostic Models in Differentiating Benign and Malignant Pulmonary Ground-Glass Nodules
Abstract
Objective: This study aimed to develop effective artificial intelligence (AI) diagnostic models based on CT images of pulmonary nodules only, on descriptional and quantitative clinical or image features, or on a combination of both to differentiate benign and malignant ground-glass nodules (GGNs) to assist in the determination of surgical intervention.
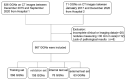
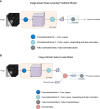
Methods: Our study included a total of 867 nodules (benign nodules: 112; malignant nodules: 755) with postoperative pathological diagnoses from two centers. For the diagnostic models to discriminate between benign and malignant GGNs, we adopted three different artificial intelligence (AI) approaches: a) an image-based deep learning approach to build a deep neural network (DNN); b) a clinical feature-based machine learning approach based on the clinical and image features of nodules; c) a fusion diagnostic model integrating the original images and the clinical and image features. The performance of the models was evaluated on an internal test dataset (the "Changzheng Dataset") and an independent test dataset collected from an external institute (the "Longyan Dataset"). In addition, the performance of automatic diagnostic models was compared with that of manual evaluations by two radiologists on the 'Longyan dataset'.
Results: The image-based deep learning model achieved an appealing diagnostic performance, yielding AUC values of 0.75 (95% confidence interval [CI]: 0.62, 0.89) and 0.76 (95% CI: 0.61, 0.90), respectively, on both the Changzheng and Longyan datasets. The clinical feature-based machine learning model performed well on the Changzheng dataset (AUC, 0.80 [95% CI: 0.64, 0.96]), whereas it performed poorly on the Longyan dataset (AUC, 0.62 [95% CI: 0.42, 0.83]). The fusion diagnostic model achieved the best performance on both the Changzheng dataset (AUC, 0.82 [95% CI: 0.71-0.93]) and the Longyan dataset (AUC, 0.83 [95% CI: 0.70-0.96]), and it achieved a better specificity (0.69) than the radiologists (0.33-0.44) on the Longyan dataset.
Conclusion: The deep learning models, including both the image-based deep learning model and the fusion model, have the ability to assist radiologists in differentiating between benign and malignant nodules for the precise management of patients with GGNs.
Keywords: artificial intelligence; computed tomography; computer-aided diagnosis; differential diagnosis; ground-glass nodule.
Copyright © 2022 Wang, Gao, Xie, Deng, Tu, Yang, Liang, Xu, Zhang, Lu, Fu, Li, Fan and Liu.
Conflict of interest statement
Authors HY, SL, PX, MZ, YL, and CF were employed by Aitrox Technology Corporation Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Multimodal Deep Learning Network for Differentiating Between Benign and Malignant Pulmonary Ground Glass Nodules.Curr Med Imaging. 2024;20:e15734056301741. doi: 10.2174/0115734056301741240903072017. Curr Med Imaging. 2024. PMID: 39257154
-
Prediction of benign and malignant ground glass pulmonary nodules based on multi-feature fusion of attention mechanism.Front Oncol. 2024 Oct 9;14:1447132. doi: 10.3389/fonc.2024.1447132. eCollection 2024. Front Oncol. 2024. PMID: 39445066 Free PMC article.
-
Computer-aided diagnosis of ground glass pulmonary nodule by fusing deep learning and radiomics features.Phys Med Biol. 2021 Mar 4;66(6):065015. doi: 10.1088/1361-6560/abe735. Phys Med Biol. 2021. PMID: 33596552
-
[Artificial intelligence research advances in discrimination and diagnosis of pulmonary ground-glass nodules].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Jun 12;47(6):566-570. doi: 10.3760/cma.j.cn112147-20231214-00370. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38858209 Review. Chinese.
-
Imaging diagnostics of pulmonary ground-glass nodules: a narrative review with current status and future directions.Quant Imaging Med Surg. 2024 Aug 1;14(8):6123-6146. doi: 10.21037/qims-24-674. Epub 2024 Jul 18. Quant Imaging Med Surg. 2024. PMID: 39144060 Free PMC article. Review.
Cited by
-
An early lung cancer diagnosis model for non-smokers incorporating ct imaging analysis and circulating genetically abnormal cells (CACs).BMC Cancer. 2025 Jan 22;25(1):124. doi: 10.1186/s12885-024-13268-5. BMC Cancer. 2025. PMID: 39844169 Free PMC article.
-
A narrative review of the clinical approach to subsolid pulmonary nodules.Ann Transl Med. 2023 Mar 15;11(5):217. doi: 10.21037/atm-22-5246. Epub 2023 Mar 10. Ann Transl Med. 2023. PMID: 37007560 Free PMC article. Review.
-
Artificial intelligence-measured nodule mass for determining the invasiveness of neoplastic ground glass nodules.Quant Imaging Med Surg. 2024 Sep 1;14(9):6698-6710. doi: 10.21037/qims-24-665. Epub 2024 Aug 28. Quant Imaging Med Surg. 2024. PMID: 39281163 Free PMC article.
-
Baseline whole-lung CT features deriving from deep learning and radiomics: prediction of benign and malignant pulmonary ground-glass nodules.Front Oncol. 2023 Aug 17;13:1255007. doi: 10.3389/fonc.2023.1255007. eCollection 2023. Front Oncol. 2023. PMID: 37664069 Free PMC article.
-
Automatic detection of adenoid hypertrophy on lateral nasopharyngeal radiographs of children based on deep learning.Transl Pediatr. 2024 Aug 31;13(8):1368-1377. doi: 10.21037/tp-24-194. Epub 2024 Aug 28. Transl Pediatr. 2024. PMID: 39263285 Free PMC article.
References
LinkOut - more resources
Full Text Sources

