Redefining the Koizumi model of mouse cerebral ischemia: A comparative longitudinal study of cerebral and retinal ischemia in the Koizumi and Longa middle cerebral artery occlusion models
- PMID: 35748043
- PMCID: PMC9580169
- DOI: 10.1177/0271678X221109873
Redefining the Koizumi model of mouse cerebral ischemia: A comparative longitudinal study of cerebral and retinal ischemia in the Koizumi and Longa middle cerebral artery occlusion models
Abstract
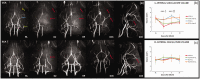
Cerebral and retinal ischemia share similar pathogenesis and epidemiology, each carrying both acute and prolonged risk of the other and often co-occurring. The most used preclinical stroke models, the Koizumi and Longa middle cerebral artery occlusion (MCAO) methods, have reported retinal damage with great variability, leaving the disruption of retinal blood supply via MCAO poorly investigated, even providing conflicting assumptions on the origin of the ophthalmic artery in rodents. The aim of our study was to use longitudinal in vivo magnetic resonance assessment of cerebral and retinal vascular perfusion after the ischemic injury to clarify whether and how the Koizumi and Longa methods induce retinal ischemia and how they differ in terms of cerebral and retinal lesion evolution. We provided anatomical evidence of the origin of the ophthalmic artery in mice from the pterygopalatine artery. Following the Koizumi surgery, retinal responses to ischemia overlapped with those in the brain, resulting in permanent damage. In contrast, the Longa method produced only extensive cerebral lesions, with greater tissue loss than in the Koizumi method. Additionally, our data suggests the Koizumi method should be redefined as a model of ischemia with chronic hypoperfusion rather than of ischemia and reperfusion.
Keywords: Brain; ischemia; magnetic resonance angiography; mice; retina.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
-
- Hepworth L, Rowe F, Walker M, et al. Post-stroke visual impairment: a systematic literature review of types and recovery of visual conditions. Ophthalmol Res 2016; 5: 1–43. doi:10.9734/or/2016/21767.
-
- Chodnicki KD, Pulido JS, Hodge DO, et al. Stroke risk before and after central retinal artery occlusion in a US cohort. Mayo Clin Proc 2019; 94: 236–241. doi:10.1016/j.mayocp.2018.10.018 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

