Reversible Structural Isomerization of Nature's Water Oxidation Catalyst Prior to O-O Bond Formation
- PMID: 35748306
- PMCID: PMC9264352
- DOI: 10.1021/jacs.2c03528
Reversible Structural Isomerization of Nature's Water Oxidation Catalyst Prior to O-O Bond Formation
Abstract
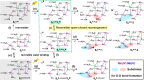
Photosynthetic water oxidation is catalyzed by a manganese-calcium oxide cluster, which experiences five "S-states" during a light-driven reaction cycle. The unique "distorted chair"-like geometry of the Mn4CaO5(6) cluster shows structural flexibility that has been frequently proposed to involve "open" and "closed"-cubane forms from the S1 to S3 states. The isomers are interconvertible in the S1 and S2 states, while in the S3 state, the open-cubane structure is observed to dominate inThermosynechococcus elongatus (cyanobacteria) samples. In this work, using density functional theory calculations, we go beyond the S3+Yz state to the S3nYz• → S4+Yz step, and report for the first time that the reversible isomerism, which is suppressed in the S3+Yz state, is fully recovered in the ensuing S3nYz• state due to the proton release from a manganese-bound water ligand. The altered coordination strength of the manganese-ligand facilitates formation of the closed-cubane form, in a dynamic equilibrium with the open-cubane form. This tautomerism immediately preceding dioxygen formation may constitute the rate limiting step for O2 formation, and exert a significant influence on the water oxidation mechanism in photosystem II.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

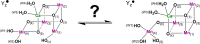



Similar articles
-
An oxyl/oxo mechanism for oxygen-oxygen coupling in PSII revealed by an x-ray free-electron laser.Science. 2019 Oct 18;366(6463):334-338. doi: 10.1126/science.aax6998. Epub 2019 Oct 17. Science. 2019. PMID: 31624207
-
The first tyrosyl radical intermediate formed in the S2-S3 transition of photosystem II.Phys Chem Chem Phys. 2014 Jun 28;16(24):11901-10. doi: 10.1039/c4cp00696h. Phys Chem Chem Phys. 2014. PMID: 24760184
-
Sequential and Coupled Proton and Electron Transfer Events in the S2 → S3 Transition of Photosynthetic Water Oxidation Revealed by Time-Resolved X-ray Absorption Spectroscopy.Biochemistry. 2016 Dec 20;55(50):6996-7004. doi: 10.1021/acs.biochem.6b01078. Epub 2016 Dec 5. Biochemistry. 2016. PMID: 27992997
-
Spin State as a Marker for the Structural Evolution of Nature's Water-Splitting Catalyst.Inorg Chem. 2016 Jan 19;55(2):488-501. doi: 10.1021/acs.inorgchem.5b02578. Epub 2015 Dec 23. Inorg Chem. 2016. PMID: 26700960 Review.
-
Biological water oxidation.Acc Chem Res. 2013 Jul 16;46(7):1588-96. doi: 10.1021/ar3003249. Epub 2013 Mar 18. Acc Chem Res. 2013. PMID: 23506074 Review.
Cited by
-
Closing Kok's cycle of nature's water oxidation catalysis.Nat Commun. 2024 Jul 16;15(1):5982. doi: 10.1038/s41467-024-50210-6. Nat Commun. 2024. PMID: 39013902 Free PMC article.
-
Conformational Flexibility of D1-Glu189: A Crucial Determinant in Substrate Water Selection, Positioning, and Stabilization within the Oxygen-Evolving Complex of Photosystem II.ACS Omega. 2024 Dec 5;9(50):50041-50048. doi: 10.1021/acsomega.4c09981. eCollection 2024 Dec 17. ACS Omega. 2024. PMID: 39713658 Free PMC article.
-
The Effect of Removal of External Proteins PsbO, PsbP and PsbQ on Flash-Induced Molecular Oxygen Evolution and Its Biphasicity in Tobacco PSII.Curr Issues Mol Biol. 2024 Jul 8;46(7):7187-7218. doi: 10.3390/cimb46070428. Curr Issues Mol Biol. 2024. PMID: 39057069 Free PMC article.
-
Electron-Transfer Route in the Early Oxidation States of the Mn4CaO5 Cluster in Photosystem II.J Phys Chem B. 2023 Jan 12;127(1):205-211. doi: 10.1021/acs.jpcb.2c08246. Epub 2022 Dec 21. J Phys Chem B. 2023. PMID: 36542840 Free PMC article.
-
Comprehensive Evaluation of Models for Ammonia Binding to the Oxygen Evolving Complex of Photosystem II.J Phys Chem B. 2024 Feb 15;128(6):1333-1349. doi: 10.1021/acs.jpcb.3c06304. Epub 2024 Feb 1. J Phys Chem B. 2024. PMID: 38299511 Free PMC article.
References
-
- Messinger J.; Renger G.. Photosynthetic water splitting. Primary Processes of Photosynthesis, Part 2: Principles and Apparatus; The Royal Society of Chemistry, 2008; Vol. 9, pp 291–349.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

