Structure-based electron-confurcation mechanism of the Ldh-EtfAB complex
- PMID: 35748623
- PMCID: PMC9232219
- DOI: 10.7554/eLife.77095
Structure-based electron-confurcation mechanism of the Ldh-EtfAB complex
Abstract
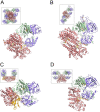
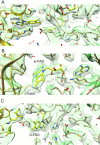
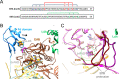
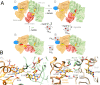
Lactate oxidation with NAD+ as electron acceptor is a highly endergonic reaction. Some anaerobic bacteria overcome the energetic hurdle by flavin-based electron bifurcation/confurcation (FBEB/FBEC) using a lactate dehydrogenase (Ldh) in concert with the electron-transferring proteins EtfA and EtfB. The electron cryo-microscopically characterized (Ldh-EtfAB)2 complex of Acetobacterium woodii at 2.43 Å resolution consists of a mobile EtfAB shuttle domain located between the rigid central Ldh and the peripheral EtfAB base units. The FADs of Ldh and the EtfAB shuttle domain contact each other thereby forming the D (dehydrogenation-connected) state. The intermediary Glu37 and Glu139 may harmonize the redox potentials between the FADs and the pyruvate/lactate pair crucial for FBEC. By integrating Alphafold2 calculations a plausible novel B (bifurcation-connected) state was obtained allowing electron transfer between the EtfAB base and shuttle FADs. Kinetic analysis of enzyme variants suggests a correlation between NAD+ binding site and D-to-B-state transition implicating a 75° rotation of the EtfAB shuttle domain. The FBEC inactivity when truncating the ferredoxin domain of EtfA substantiates its role as redox relay. Lactate oxidation in Ldh is assisted by the catalytic base His423 and a metal center. On this basis, a comprehensive catalytic mechanism of the FBEC process was proposed.
Keywords: biochemistry; bioenergetics; chemical biology; cryo-EM structure; electron bifurcation; electron-transferring flavoprotein; flavin; lactate dehydrogenase.
© 2022, Kayastha, Katsyv et al.
Conflict of interest statement
KK, AK, CH, SW, JS, UE, VM No competing interests declared
Figures













Similar articles
-
A novel mode of lactate metabolism in strictly anaerobic bacteria.Environ Microbiol. 2015 Mar;17(3):670-7. doi: 10.1111/1462-2920.12493. Epub 2014 May 21. Environ Microbiol. 2015. PMID: 24762045
-
Modulations of the reduction potentials of flavin-based electron bifurcation complexes and semiquinone stabilities are key to control directional electron flow.FEBS J. 2021 Feb;288(3):1008-1026. doi: 10.1111/febs.15343. Epub 2020 May 21. FEBS J. 2021. PMID: 32329961
-
Flavin-Based Electron Bifurcation, Ferredoxin, Flavodoxin, and Anaerobic Respiration With Protons (Ech) or NAD+ (Rnf) as Electron Acceptors: A Historical Review.Front Microbiol. 2018 Mar 14;9:401. doi: 10.3389/fmicb.2018.00401. eCollection 2018. Front Microbiol. 2018. PMID: 29593673 Free PMC article. Review.
-
The semiquinone swing in the bifurcating electron transferring flavoprotein/butyryl-CoA dehydrogenase complex from Clostridium difficile.Nat Commun. 2017 Nov 17;8(1):1577. doi: 10.1038/s41467-017-01746-3. Nat Commun. 2017. PMID: 29146947 Free PMC article.
-
Flavin-Based Electron Bifurcation, A New Mechanism of Biological Energy Coupling.Chem Rev. 2018 Apr 11;118(7):3862-3886. doi: 10.1021/acs.chemrev.7b00707. Epub 2018 Mar 21. Chem Rev. 2018. PMID: 29561602 Review.
Cited by
-
An electron-bifurcating "plug" to a protein nanowire in tungsten-dependent aldehyde detoxification.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2501900122. doi: 10.1073/pnas.2501900122. Epub 2025 Jul 22. Proc Natl Acad Sci U S A. 2025. PMID: 40694326
-
Noncovalent interactions that tune the reactivities of the flavins in bifurcating electron transferring flavoprotein.J Biol Chem. 2023 Jun;299(6):104762. doi: 10.1016/j.jbc.2023.104762. Epub 2023 Apr 27. J Biol Chem. 2023. PMID: 37119850 Free PMC article.
-
Molecular architecture and electron transfer pathway of the Stn family transhydrogenase.Nat Commun. 2023 Sep 7;14(1):5484. doi: 10.1038/s41467-023-41212-x. Nat Commun. 2023. PMID: 37673911 Free PMC article.
-
AlphaFold2 and its applications in the fields of biology and medicine.Signal Transduct Target Ther. 2023 Mar 14;8(1):115. doi: 10.1038/s41392-023-01381-z. Signal Transduct Target Ther. 2023. PMID: 36918529 Free PMC article. Review.
-
Lactate dehydrogenase D is a general dehydrogenase for D-2-hydroxyacids and is associated with D-lactic acidosis.Nat Commun. 2023 Oct 20;14(1):6638. doi: 10.1038/s41467-023-42456-3. Nat Commun. 2023. PMID: 37863926 Free PMC article.
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica. Section D, Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Bock AK, Prieger-Kraft A, Schönheit P. Pyruvate? a novel substrate for growth and methane formation in Methanosarcina barkeri. Archives of Microbiology. 1994;161:33–46. doi: 10.1007/s002030050019. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

