Decreased Antibody Response After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in Patients With Down Syndrome
- PMID: 35748853
- PMCID: PMC9278229
- DOI: 10.1093/infdis/jiac235
Decreased Antibody Response After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in Patients With Down Syndrome
Abstract
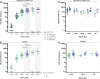
The risk of a severe course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in adults with Down syndrome is increased, resulting in an up to 10-fold increase in mortality, in particular in those >40 years of age. After primary SARS-CoV-2 vaccination, the higher risks remain. In this prospective observational cohort study, SARS-CoV-2 spike S1-specific antibody responses after routine SARS-CoV-2 vaccination (BNT162b2, messenger RNA [mRNA]-1273, or ChAdOx1) in adults with Down syndrome and healthy controls were compared. Adults with Down syndrome showed lower antibody concentrations after 2 mRNA vaccinations or after 2 ChAdOx1 vaccinations. After 2 mRNA vaccinations, lower antibody concentrations were seen with increasing age.
Clinical trials registration: NCT05145348.
Keywords: COVID-19 vaccination; Down syndrome; SARS-CoV-2; antibody response.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

Comment in
-
Defective Immune Response to SARS-CoV-2 Immunization in Down Syndrome Correlates With Increased Susceptibility to Severe Illness With Infection.J Infect Dis. 2022 Sep 13;226(5):755-756. doi: 10.1093/infdis/jiac237. J Infect Dis. 2022. PMID: 35749348 Free PMC article. No abstract available.
References
-
- Hüls A, Costa ACS, Dierssen M, et al. . An international survey on the impact of COVID-19 in individuals with Down syndrome. medRxiv [Preprint]. Posted online 5 November 2020. doi:10.1101/2020.11.03.20225359. - DOI
-
- Meier S, Bel M, L’Huillier A, et al. . Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. Vaccine 2011; 29:3548–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

