Luminescence and Length Control in Nonchelated d8 -Metallosupramolecular Polymers through Metal-Metal Interactions
- PMID: 35749048
- PMCID: PMC9545304
- DOI: 10.1002/anie.202208436
Luminescence and Length Control in Nonchelated d8 -Metallosupramolecular Polymers through Metal-Metal Interactions
Abstract
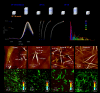
Supramolecular polymers (SPs) of d8 transition metal complexes have received considerable attention by virtue of their rich photophysical properties arising from metal-metal interactions. However, thus far, the molecular design is restricted to complexes with chelating ligands due to their advantageous preorganization and strong ligand fields. Herein, we demonstrate unique pathway-controllable metal-metal-interactions and remarkable 3 MMLCT luminescence in SPs of a non-chelated PtII complex. Under kinetic control, self-complementary bisamide H-bonding motifs induce a rapid self-assembly into non-emissive H-type aggregates (1A). However, under thermodynamic conditions, a more efficient ligand coplanarization leads to superiorly stabilized SP 1B with extended Pt⋅⋅⋅Pt interactions and remarkably long 3 MMLCT luminescence (τ77 K =0.26 ms). The metal-metal interactions could be subsequently exploited to control the length of the emissive SPs using the seeded-growth approach.
Keywords: hydrogen bonding; luminescent assemblies; metal-metal-interactions; pathway complexity; supramolecular polymers.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

