Serial T-SPOT.TB responses in Tanzanian adolescents: Transient, persistent and irregular conversions
- PMID: 35749397
- PMCID: PMC9231806
- DOI: 10.1371/journal.pone.0268685
Serial T-SPOT.TB responses in Tanzanian adolescents: Transient, persistent and irregular conversions
Abstract
Background: Prospective studies of interferon-gamma release assays (IGRA) on healthy subjects in tuberculosis-endemic regions have not examined the long-term variability of serial assays. This issue is relevant to the interpretation of tuberculosis (TB) vaccine trials based on prevention of infection.
Methods: T-SPOT.TB assays were performed manually on healthy adolescents during a tuberculosis vaccine trial in Tanzania at 5 intervals over 3 years. Assay results were defined as negative, positive, borderline or invalid. Subsequently, microtiter plates were analyzed by an automated reader to obtain quantitative counts of spot forming cells (SFCs) for the present analysis.
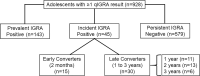
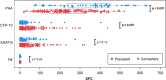
Results: 3387 T-SPOT.TB samples were analyzed from 928 adolescents; manual and automated assay results were 97% concordant. Based on the quantitative results 143 (15%) participants were prevalent IGRA-positives at baseline, were ineligible for further study. Among the remaining IGRA-negative participants, the annual rate of IGRA conversion was 2·9%. Among 43 IGRA converters with repeat assays 12 (28%) were persistent converters, 16 (37%) were transient converters, and 15 (35%) comprised a new category defined as irregular converters (≥2 different subsequent results). ESAT-6 and CFP-10 responses were higher in prevalent than incident positives: 53 vs 36 for CFP-10 (p < 0·007); 44 vs 34 for ESAT-6 (p = 0·12).
Conclusions: Definitions of IGRA conversion, reversion, and persistence depend critically on the frequency of testing. Multiple shifts in categories among adolescents in a TB-endemic country may represent multiple infections, variable host responses in subclinical infection, or assay variation. These findings should to be considered in the design and interpretation of TB vaccine trials based on prevention of infection. Household contact studies could determine whether even transient IGRA conversion might represent exposure to an active case of M. tuberculosis disease.
Conflict of interest statement
The authors have declared that no competing interests exists.
Figures
References
-
- World Health Organisation. Latent tuberculosis infection: Updtaed and consolidtaed guidelines for programmatic management 2018. [Available from: https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. - PubMed
-
- Lu P, Liu Q, Zhou Y, Martinez L, Kong W, Ding X, et al.. Predictors of Discordant Tuberculin Skin test and QuantiFERON-TB Gold In-Tube Results in Eastern China: A Population-based, Cohort Study. Clin Infect Dis. 2020. - PubMed
-
- Abubakar I, Drobniewski F, Southern J, Sitch AJ, Jackson C, Lipman M, et al.. Prognostic value of interferon-gamma release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis. 2018;18(10):1077–87. doi: 10.1016/S1473-3099(18)30355-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

