Novel insights on [1,2]oxazolo[5,4-e]isoindoles on multidrug resistant acute myeloid leukemia cell line
- PMID: 35749723
- PMCID: PMC9540667
- DOI: 10.1002/ddr.21962
Novel insights on [1,2]oxazolo[5,4-e]isoindoles on multidrug resistant acute myeloid leukemia cell line
Abstract
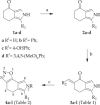
A series of [1,2]oxazolo[5,4-e]isoindole derivatives was evaluated against HL-60 cell line and its multidrug resistance (MDR) variant, HL-60R, resistant to doxorubicin and to other P-gp substrates by overexpressing the efflux pump. They displayed antiproliferative activities, with IC50 values ranging from 0.02 to 5.5 µM. In particular, the newly synthesized compound 4k produced synergistic effects in terms of cell growth inhibition and cell death induction either in combination with a Vinca alkaloid, Vinblastine, and a Taxane, Paclitaxel in HL-60R cells. The study of the mechanism of action indicated that all compounds showed antimitotic activity through inhibition of tubulin polymerization. Thus, [1,2]oxazoles could represent a valuable tool to overcome MDR mechanism, confirming the potential use of this class of compounds.
Keywords: [1,2]oxazolo[5,4-e]isoindoles; antimitotic agents; multidrug resistance.
© 2022 The Authors. Drug Development Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[1,2]Oxazolo[5,4-e]isoindoles as promising tubulin polymerization inhibitors.Eur J Med Chem. 2016 Nov 29;124:840-851. doi: 10.1016/j.ejmech.2016.09.013. Epub 2016 Sep 5. Eur J Med Chem. 2016. PMID: 27643641
-
A thalidomide analogue with in vitro antiproliferative, antimitotic, and microtubule-stabilizing activities.Mol Cancer Ther. 2006 Feb;5(2):450-6. doi: 10.1158/1535-7163.MCT-05-0254. Mol Cancer Ther. 2006. PMID: 16505120
-
Modulation of the spacer in N,N-bis(alkanol)amine aryl ester heterodimers led to the discovery of a series of highly potent P-glycoprotein-based multidrug resistance (MDR) modulators.Eur J Med Chem. 2019 Jun 15;172:71-94. doi: 10.1016/j.ejmech.2019.03.054. Epub 2019 Mar 27. Eur J Med Chem. 2019. PMID: 30947123
-
Modulation of multidrug resistance (MDR) in hematological malignancies.Ann Oncol. 1999;10 Suppl 6:53-9. Ann Oncol. 1999. PMID: 10676553 Review.
-
Therapeutic strategies to overcome taxane resistance in cancer.Drug Resist Updat. 2021 Mar;55:100754. doi: 10.1016/j.drup.2021.100754. Epub 2021 Feb 27. Drug Resist Updat. 2021. PMID: 33691261 Review.
Cited by
-
Synthesis of Imidazo[2,1-a]isoindolones via Rearrangement and Tandem Cyclization of Amino-Acid-Based N-Phenacyl-2-cyano-4-nitrobenzensulfonamides.J Org Chem. 2025 Jun 6;90(22):7151-7160. doi: 10.1021/acs.joc.4c03113. Epub 2025 May 27. J Org Chem. 2025. PMID: 40420460 Free PMC article.
-
Deregulated Gene Expression Profiles and Regulatory Networks in Adult and Pediatric RUNX1/RUNX1T1-Positive AML Patients.Cancers (Basel). 2023 Mar 16;15(6):1795. doi: 10.3390/cancers15061795. Cancers (Basel). 2023. PMID: 36980682 Free PMC article.
-
Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives.Pharmaceuticals (Basel). 2023 Mar 11;16(3):429. doi: 10.3390/ph16030429. Pharmaceuticals (Basel). 2023. PMID: 36986528 Free PMC article.
-
HSV-1 Glycoprotein D and Its Surface Receptors: Evaluation of Protein-Protein Interaction and Targeting by Triazole-Based Compounds through In Silico Approaches.Int J Mol Sci. 2023 Apr 11;24(8):7092. doi: 10.3390/ijms24087092. Int J Mol Sci. 2023. PMID: 37108255 Free PMC article.
-
MiR-133 promotes the multidrug resistance of acute myeloid leukemia cells (HL-60/ADR) to daunorubicin.Cytotechnology. 2024 Dec;76(6):833-846. doi: 10.1007/s10616-024-00656-9. Epub 2024 Sep 19. Cytotechnology. 2024. PMID: 39435426
References
-
- Aapro, M. S. , Alberts, D. S. , & Salmon, S. E. (1983). Interaction of human leukocyte interferon with vinca alkaloids and other chemotherapeutic agents against human tumors in clonogenic assay. Cancer Chemotherapy and Pharmacology, 10, 161–168. - PubMed
-
- Aggarwal, B. B. , Shishodia, S. , Takada, Y. , Banerjee, S. , Newman, R. A. , Bueso‐Ramos, C. E. , & Price, J. E. (2005). Curcumin suppresses the paclitaxel‐induced nuclear factor‐ΚB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clinical Cancer Research, 11(20), 7490–7498. - PubMed
-
- Arribas, A. , Napoli, S. , Cascione, L. , Gaudio, E. , Bordone‐Pittau, R. , Barreca, M. , Sartori, G. , Chiara, T. , Spriano, F. , Rinaldi, A. , Stathis, A. , Stussi, G. , Rossi, D. , Emanuele, Z. , & Bertoni, F. (2020). Secondary resistance to the PI3K inhibitor copanlisib in marginal zone lymphoma. European Journal of Cancer, 138, S40.
-
- Assaraf, Y. G. , Brozovic, A. , Gonçalves, A. C. , Jurkovicova, D. , Linē, A. , Machuqueiro, M. , Saponara, S. , Sarmento‐Ribeiro, A. B. , Xavier, C. P. R. , & Vasconcelos, M. H. (2019). The multi‐factorial nature of clinical multidrug resistance in cancer. Drug Resistance Updates, 46(August), 100645. - PubMed
-
- Barraja, P. , Spanò, V. , Diana, P. , Carbone, A. , Cirrincione, G. , Vedaldi, D. , Salvador, A. , Viola, G. , & Dall'Acqua, F. (2009). Pyrano[2,3‐e]Isoindol‐2‐Ones, new angelicin heteroanalogues. Bioorganic & Medicinal Chemistry Letters, 19(6), 1711–1714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

