The Sleep Well Baby project: an automated real-time sleep-wake state prediction algorithm in preterm infants
- PMID: 35749799
- PMCID: PMC9548667
- DOI: 10.1093/sleep/zsac143
The Sleep Well Baby project: an automated real-time sleep-wake state prediction algorithm in preterm infants
Abstract
Study objectives: Sleep is an important driver of early brain development. However, sleep is often disturbed in preterm infants admitted to the neonatal intensive care unit (NICU). We aimed to develop an automated algorithm based on routinely measured vital parameters to classify sleep-wake states of preterm infants in real-time at the bedside.
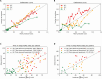
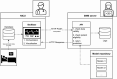
Methods: In this study, sleep-wake state observations were obtained in 1-minute epochs using a behavioral scale developed in-house while vital signs were recorded simultaneously. Three types of vital parameter data, namely, heart rate, respiratory rate, and oxygen saturation, were collected at a low-frequency sampling rate of 0.4 Hz. A supervised machine learning workflow was used to train a classifier to predict sleep-wake states. Independent training (n = 37) and validation datasets were validation n = 9) datasets were used. Finally, a setup was designed for real-time implementation at the bedside.
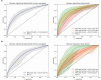
Results: The macro-averaged area-under-the-receiver-operator-characteristic (AUROC) of the automated sleep staging algorithm ranged between 0.69 and 0.82 for the training data, and 0.61 and 0.78 for the validation data. The algorithm provided the most accurate prediction for wake states (AUROC = 0.80). These findings were well validated on an independent sample (AUROC = 0.77).
Conclusions: With this study, to the best of our knowledge, a reliable, nonobtrusive, and real-time sleep staging algorithm was developed for the first time for preterm infants. Deploying this algorithm in the NICU environment may assist and adapt bedside clinical work based on infants' sleep-wake states, potentially promoting the early brain development and well-being of preterm infants.
Keywords: automated sleep staging; machine learning; neonatal intensive care; preterm; sleep.
© Sleep Research Society 2022. Published by Oxford University Press on behalf of the Sleep Research Society.
Figures