Genetic variants involved in the cGAS-STING pathway predict outcome in patients with metastatic colorectal cancer: Data from FIRE-3 and TRIBE trials
- PMID: 35749909
- PMCID: PMC11970509
- DOI: 10.1016/j.ejca.2022.05.016
Genetic variants involved in the cGAS-STING pathway predict outcome in patients with metastatic colorectal cancer: Data from FIRE-3 and TRIBE trials
Abstract
Background: The activation of stimulator of interferon genes (STING) was reported to enhance cetuximab-mediated natural killer cell activation and dendritic cell maturation. Polymorphisms in genes in the cyclic GMP-AMP synthase (cGAS)-STING pathway may affect innate immune response. Therefore, we hypothesised that genetic variants in the cGAS-STING pathway may predict the efficacy of cetuximab-based treatment in patients with metastatic colorectal cancer.
Methods: Genomic DNA from blood samples of patients enrolled in FIRE-3 (cetuximab arm, n = 129; bevacizumab arm, n = 107) and TRIBE (bevacizumab arm, n = 215) was genotyped using the OncoArray-500K bead chip panel. Seven selected single nucleotide polymorphisms in 3 genes (cGAS, STING and interferon B1 (IFNB1)) were analysed for the association with overall survival and progression-free survival.
Results: In the cetuximab cohort, patients with STING rs1131769 any T allele showed significantly shorter overall survival (36.3 versus 56.1 months) than carriers of C/C in both univariate [hazard ratio (HR) = 2.08; 95% confidence interval (CI): 1.06-4.07; P = 0.03] and multivariate (HR = 2.98; 95% CI: 1.35-6.6; P = 0.0085) analyses; patients carrying IFNB1 rs1051922 G/A and A/A genotype showed a significantly shorter progression-free survival than carriers of G/G allele in both univariate (G/A versus G/G, 10.2 versus 14.1 months, HR = 1.84; 95% CI 1.23-2.76; A/A versus G/G, 10.7 versus 14.1 months, HR = 2.19; 95% CI 0.97-4.96; P = 0.0077) and multivariate analyses (G/A versus G/G, HR = 2; 95% CI 1.22-3.3; A/A versus G/G, HR = 2.19, 95% CI 0.92-5.26, P = 0.02). These associations were not observed in the bevacizumab arm of FIRE-3 or TRIBE.
Conclusion: These results suggest for the first time that germline polymorphisms in STING and IFNB1 genes may predict the outcomes of cetuximab-based treatment in patients with metastatic colorectal cancer.
Keywords: Biomarker; Cetuximab; Colorectal cancer; STING.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement H.-J.L. reports receiving honoraria from consultant/advisory board membership for Merck Serono, Bayer, and Genentech. S.S. reports receiving honoraria for talks and advisory board role: AMGEN, Bayer, BMS, Pierre-Fabre, Merck KGaA; MSD, Leo-Pharma, Lilly, Sanofi, Servier, Roche, Takeda, Taiho. A.F reports receiving honoraria as consultant/advisory board for Amgen, Bayer, Bristol, Daiichi Sankyo, Incyte, Lilly, Merck, MSD, Pierre-Fabre, Roche, Servier. All remaining authors have declared no conflicts of interest.
Figures
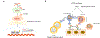


References
-
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28(8):1713–29. 10.1093/annonc/mdx175. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

