Outcomes of flucytosine-containing combination treatment for cryptococcal meningitis in a South African national access programme: a cross-sectional observational study
- PMID: 35750065
- PMCID: PMC11334497
- DOI: 10.1016/S1473-3099(22)00234-1
Outcomes of flucytosine-containing combination treatment for cryptococcal meningitis in a South African national access programme: a cross-sectional observational study
Erratum in
-
Correction to Lancet Infect Dis 2022; published online June 21. https://doi.org/10.1016/S1473-3099(22)00234-1.Lancet Infect Dis. 2022 Aug;22(8):e207. doi: 10.1016/S1473-3099(22)00437-6. Epub 2022 Jun 30. Lancet Infect Dis. 2022. PMID: 35779547 No abstract available.
-
Correction to Lancet Infect Dis 2022; 22: 1365-73.Lancet Infect Dis. 2022 Nov;22(11):e310. doi: 10.1016/S1473-3099(22)00588-6. Epub 2022 Sep 5. Lancet Infect Dis. 2022. PMID: 36075231 No abstract available.
Abstract
Background: Although flucytosine is a key component of WHO-recommended induction treatment for HIV-associated cryptococcal meningitis, this antifungal agent is not widely available in low-income and middle-income countries due to limited production and cost. In 2018, a national flucytosine access programme was initiated in South Africa. We aimed to determine the effectiveness of flucytosine-containing induction regimens in routine care to motivate for the urgent registration of flucytosine and its inclusion in treatment guidelines.
Methods: In this cross-sectional study, we compared outcomes of adults aged 18 years and older with incident laboratory-confirmed cryptococcal meningitis treated with or without flucytosine-containing regimens at 19 sentinel hospitals in South Africa. A case of cryptococcosis was defined as illness in an adult with: (1) positive cerebrospinal fluid (CSF) India ink microscopy; (2) a positive CSF cryptococcal antigen test; or (3) culture of Cryptococcus neoformans or Cryptococcus gattii from CSF or any other specimen. We excluded patients without a case report form, those with an unknown or negative HIV serology result, those with a recurrent episode, and those who did not receive antifungal treatment in hospital. We assessed cumulative in-hospital mortality at 14 days and 30 days and calculated the overall crude in-hospital case-fatality ratio. We used random-effects logistic regression to examine the association between treatment group and in-hospital mortality.
Findings: From July 1, 2018, to March 31, 2020, 10 668 individuals were diagnosed with laboratory-confirmed cryptococcal meningitis, 7787 cases diagnosed at non-enhanced surveillance sites and 567 cases from eight enhanced surveillance sites with no access to flucytosine were excluded. Of 2314 adults with a first episode of cryptococcosis diagnosed at 19 facilities with access to flucytosine, 1996 had a case report form and of these, 1539 received induction antifungal treatment and were confirmed HIV-seropositive first-episode cases. Of 1539 patients who received antifungal therapy, 596 (38·7%) individuals received a flucytosine-containing regimen and 943 (61·3%) received another regimen. The median age was 36 years (IQR 32-43) and 906 (58·9%) participants were male and 633 (41·1%) were female. The crude in-hospital case-fatality ratio was 23·9% (95% CI 20·0-27·0; 143 of 596) in those treated with flucytosine-containing regimens and 37·2% (95% CI 34·0-40·0; 351 of 943) in those treated with other regimens. Patients admitted to non-academic hospitals (adjusted odds ratio [aOR] 1·95 [95% CI 1·53-2·48]; p<0·0001) and those who were antiretroviral treatment-experienced (aOR 1·30 [1·02-1·67]; p=0·033) were more likely to receive flucytosine. After adjusting for relevant confounders, flucytosine treatment was associated with a 53% reduction in mortality (aOR 0·47 [95% CI 0·35-0·64]; p<0·0001). Among survivors, the median length of hospital admission in the flucytosine group was 11 days (IQR 8-15) versus 17 days (13-21) in the comparison group (p=0·0010).
Interpretation: In-hospital mortality among patients treated with a flucytosine-containing regimen was comparable to reduced mortality reported in patients receiving a flucytosine-containing regimen in a recent multicentre African clinical trial. Flucytosine-based treatment can be delivered in routine care in a middle-income country with a substantial survival benefit.
Funding: National Institute for Communicable Diseases, a Division of the National Health Laboratory Service.
Translation: For the Zulu translation of the abstract see Supplementary Materials section.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests NPG was partly supported by a National Institutes of Health grant (1R01AI118511-01A1). GM was supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (grant number, 64787). STM reports a grant from Sanofi (grant number, MEN00041). All other authors declare no competing interests.
Figures

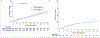
Comment in
-
Access to flucytosine for the treatment of HIV-associated cryptococcal meningitis in Africa.Lancet Infect Dis. 2022 Sep;22(9):1262-1264. doi: 10.1016/S1473-3099(22)00315-2. Epub 2022 Jun 21. Lancet Infect Dis. 2022. PMID: 35750066 No abstract available.
References
-
- World Health Organization (WHO). Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. World Health Organization. 2018. https://apps.who.int/iris/bitstream/handle/10665/260399/9789241550277-en.... (assessed January 13, 2021). - PubMed
-
- Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa. New England Journal of Medicine. 2018;378:1004–1017. - PubMed
-
- Application for WHO Model List of Essential Medicines: Flucytosine (5FC). 2018. http://www.life-worldwide.org/assets/uploads/files/Flucytosine_63_CORE_A.... (assessed February 17, 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

