Preferential Gs protein coupling of the galanin Gal1 receptor in the µ-opioid-Gal1 receptor heterotetramer
- PMID: 35750299
- PMCID: PMC9462584
- DOI: 10.1016/j.phrs.2022.106322
Preferential Gs protein coupling of the galanin Gal1 receptor in the µ-opioid-Gal1 receptor heterotetramer
Abstract
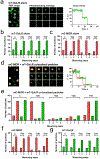
Recent studies have proposed that heteromers of µ-opioid receptors (MORs) and galanin Gal1 receptors (Gal1Rs) localized in the mesencephalon mediate the dopaminergic effects of opioids. The present study reports converging evidence, using a peptide-interfering approach combined with biophysical and biochemical techniques, including total internal reflection fluorescence microscopy, for a predominant homodimeric structure of MOR and Gal1R when expressed individually, and for their preference to form functional heterotetramers when co-expressed. Results show that a heteromerization-dependent change in the Gal1R homodimeric interface leads to a switch in G-protein coupling from inhibitory Gi to stimulatory Gs proteins. The MOR-Gal1R heterotetramer, which is thus bound to Gs via the Gal1R homodimer and Gi via the MOR homodimer, provides the framework for a canonical Gs-Gi antagonist interaction at the adenylyl cyclase level. These novel results shed light on the intense debate about the oligomeric quaternary structure of G protein-coupled receptors, their predilection for heteromer formation, and the resulting functional significance.
Keywords: DAMGO (PubChem CID: 5462471); Endomorphin-1 (PubChem CID: 5311080); Fentanyl (PubChem CID: 3345); G protein coupled receptor oligomerization; Galanin (PubChem CID: 16133823); Galanin receptors; M40 (PubChem CID: 16133821); M617 (PubChem CID: 16158157); Methadone (PubChem CID: 4095); Naloxone (PubChem CID: 5284596); Opioid receptors; Total internal reflection fluorescence microscopy.
Published by Elsevier Ltd.
Figures


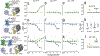


References
-
- Asher WB, Mathiasen S, Holsey MD, Grinnell SG, Lambert NA, Javitch JA, Extreme vetting of dopamine receptor oligomerization, in: Herrick-Davis K, Milligan G, Di Giovanni G (Eds.), G-Protein-Coupled Receptor Dimers, Springer, Switzerland, 99–127 (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

