Tumour immune microenvironment in resected thymic carcinomas as a predictor of clinical outcome
- PMID: 35750748
- PMCID: PMC9470747
- DOI: 10.1038/s41416-022-01875-7
Tumour immune microenvironment in resected thymic carcinomas as a predictor of clinical outcome
Abstract
Background: The spatial distribution of tumour-infiltrating lymphocytes (TILs) is a novel descriptor characterising the tumour immune microenvironment (TIME). The aim of our study was to assess whether a specific TIME of surgically resected thymic carcinoma (TC) can predict tumour invasiveness, recurrence or survival.
Methods: Digital microscopy was performed on 39 TCs immunohistochemically stained to investigate the activation of the immune checkpoint pathway (PD-L1/PD-1), along with density and spatial distribution of TILs phenotypes (CD3+, CD4+, CD8+, FOXP3+, CD56+). The impact of PD-L1 and TIL density considering the intratumoural (iTILs) and stromal (sTILs) distribution on pathological characteristics and clinical outcomes were analysed.
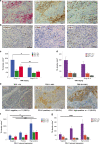
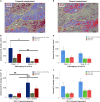
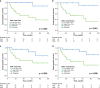
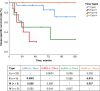
Results: In early TC stages, we observed a higher total density of CD3+ (p = 0.05) and CD8+ (p = 0.02) TILs. PD-L1 was expressed in 71.8% of TCs. In advanced TC stages, we observed a lower density of CD3+ (p = 0.04) and CD8+ (p = 0.01) iTILs compared to early stages. Serum concentrations of PD-L1 were significantly higher in TCs compared to healthy controls: 134.43 ± 18.51 vs. 82.01 ± 6.34 pg/ml (p = 0.001), respectively. High densities of stromal CD4+ TILs (54 vs. 32%, p = 0.043) and CD8+ TILs (65 vs. 17%, p = 0.048) were associated with improved freedom from recurrence (FFR) and cause-specific survival (CSS). High density of FoxP3+ TILs were associated with improved FFR (p = 0.03) and CSS (p = 0.003).
Discussion: Mapping TIL subpopulations complement the armamentarium for prognostication of TC outcomes. The improved outcome in patients with high density of TILs supports the use of immune checkpoint inhibitors in TC patients.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Marx A, Ströbel P, Badve SS, Chalabreysse L, Chan JK, Chen G, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9:596–611. doi: 10.1097/JTO.0000000000000154. - DOI - PubMed
-
- Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S65–72. doi: 10.1097/JTO.0000000000000290. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

