Screening strategies and laboratory assays to support Plasmodium falciparum histidine-rich protein deletion surveillance: where we are and what is needed
- PMID: 35751070
- PMCID: PMC9233320
- DOI: 10.1186/s12936-022-04226-2
Screening strategies and laboratory assays to support Plasmodium falciparum histidine-rich protein deletion surveillance: where we are and what is needed
Erratum in
-
Correction: Screening strategies and laboratory assays to support Plasmodium falciparum histidine-rich protein deletion surveillance: where we are and what is needed.Malar J. 2022 Sep 14;21(1):266. doi: 10.1186/s12936-022-04281-9. Malar J. 2022. PMID: 36104721 Free PMC article. No abstract available.
Abstract
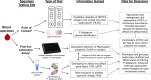
Rapid diagnostic tests (RDTs) detecting Plasmodium falciparum histidine-rich protein 2 (HRP2) have been an important tool for malaria diagnosis, especially in resource-limited settings lacking quality microscopy. Plasmodium falciparum parasites with deletion of the pfhrp2 gene encoding this antigen have now been identified in dozens of countries across Asia, Africa, and South America, with new reports revealing a high prevalence of deletions in some selected regions. To determine whether HRP2-based RDTs are appropriate for continued use in a locality, focused surveys and/or surveillance activities of the endemic P. falciparum population are needed. Various survey and laboratory methods have been used to determine parasite HRP2 phenotype and pfhrp2 genotype, and the data collected by these different methods need to be interpreted in the appropriate context of survey and assay utilized. Expression of the HRP2 antigen can be evaluated using point-of-care RDTs or laboratory-based immunoassays, but confirmation of a deletion (or mutation) of pfhrp2 requires more intensive laboratory molecular assays, and new tools and strategies for rigorous but practical data collection are particularly needed for large surveys. Because malaria diagnostic strategies are typically developed at the national level, nationally representative surveys and/or surveillance that encompass broad geographical areas and large populations may be required. Here is discussed contemporary assays for the phenotypic and genotypic evaluation of P. falciparum HRP2 status, consider their strengths and weaknesses, and highlight key concepts relevant to timely and resource-conscious workflows required for efficient diagnostic policy decision making.
Keywords: Gene deletions; Histidine-rich protein; Laboratory assay; Malaria; Rapid diagnostic test; Surveillance; pfhrp2; pfhrp3.
© 2022. The Author(s).
Conflict of interest statement
JBP reports having received research support from the WHO and NIH relevant to the scope of this work. JBP also reports research support from Gilead Sciences, non-financial support from Abbott Laboratories, and an honorarium from Virology Education for studies of viral hepatitis and COVID-19, outside the scope of the current work.
Figures
References
-
- WHO . Guidelines for malaria. Geneva: World Health Organization; 2021. - PubMed
-
- WHO . Malaria rapid diagnostic test performance Results of WHO product testing of malaria RDTs: Round 8 (2016–2018) Geneva: World Health Organization; 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

