Utilization of lignocellulosic biofuel conversion residue by diverse microorganisms
- PMID: 35751080
- PMCID: PMC9233362
- DOI: 10.1186/s13068-022-02168-0
Utilization of lignocellulosic biofuel conversion residue by diverse microorganisms
Abstract
Background: Lignocellulosic conversion residue (LCR) is the material remaining after deconstructed lignocellulosic biomass is subjected to microbial fermentation and treated to remove the biofuel. Technoeconomic analyses of biofuel refineries have shown that further microbial processing of this LCR into other bioproducts may help offset the costs of biofuel generation. Identifying organisms able to metabolize LCR is an important first step for harnessing the full chemical and economic potential of this material. In this study, we investigated the aerobic LCR utilization capabilities of 71 Streptomyces and 163 yeast species that could be engineered to produce valuable bioproducts. The LCR utilization by these individual microbes was compared to that of an aerobic mixed microbial consortium derived from a wastewater treatment plant as representative of a consortium with the highest potential for degrading the LCR components and a source of genetic material for future engineering efforts.
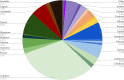
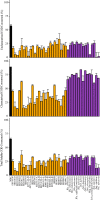
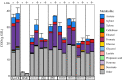
Results: We analyzed several batches of a model LCR by chemical oxygen demand (COD) and chromatography-based assays and determined that the major components of LCR were oligomeric and monomeric sugars and other organic compounds. Many of the Streptomyces and yeast species tested were able to grow in LCR, with some individual microbes capable of utilizing over 40% of the soluble COD. For comparison, the maximum total soluble COD utilized by the mixed microbial consortium was about 70%. This represents an upper limit on how much of the LCR could be valorized by engineered Streptomyces or yeasts into bioproducts. To investigate the utilization of specific components in LCR and have a defined media for future experiments, we developed a synthetic conversion residue (SynCR) to mimic our model LCR and used it to show lignocellulose-derived inhibitors (LDIs) had little effect on the ability of the Streptomyces species to metabolize SynCR.
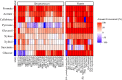
Conclusions: We found that LCR is rich in carbon sources for microbial utilization and has vitamins, minerals, amino acids and other trace metabolites necessary to support growth. Testing diverse collections of Streptomyces and yeast species confirmed that these microorganisms were capable of growth on LCR and revealed a phylogenetic correlation between those able to best utilize LCR. Identification and quantification of the components of LCR enabled us to develop a synthetic LCR (SynCR) that will be a useful tool for examining how individual components of LCR contribute to microbial growth and as a substrate for future engineering efforts to use these microorganisms to generate valuable bioproducts.
Keywords: Biofuel; Conversion residue; Lignocellulose; Streptomyces; Valorization; Yeasts.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Ng RTL, Fasahati P, Huang K, Maravelias CT. Utilizing stillage in the biorefinery: economic, technological and energetic analysis. Appl Energ. 2019;241:491–503. doi: 10.1016/j.apenergy.2019.03.020. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
