The kinetics of IgG subclasses and contributions to neutralizing activity against SARS-CoV-2 wild-type strain and variants in healthy adults immunized with inactivated vaccine
- PMID: 35751471
- PMCID: PMC9349727
- DOI: 10.1111/imm.13531
The kinetics of IgG subclasses and contributions to neutralizing activity against SARS-CoV-2 wild-type strain and variants in healthy adults immunized with inactivated vaccine
Abstract
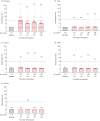
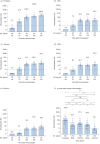
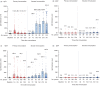
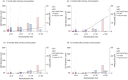
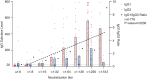
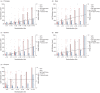
Neutralizing antibody is an important indicator of vaccine efficacy, of which IgG is the main component. IgG can be divided into four subclasses. Up to now, studies analysing the humoral response to SARS-CoV-2 vaccination have mostly focused on measuring total IgG, and the contribution of specific IgG subclasses remains elusive. The aim of this study is to investigate the kinetics of neutralizing antibodies and IgG subclasses, and to explore their relationships in people vaccinated with inactivated COVID-19 vaccine. We conducted a prospective cohort study in 174 healthy adults aged 18-59 years old who were administrated 2 doses of CoronaVac 14 days apart and a booster dose 1 year after the primary immunization, and followed up for 15 months. Blood samples were collected at various time points after primary and booster immunization. We used live SARS-CoV-2 virus neutralizing assay to determine neutralizing ability against the wild-type strain and 4 variants (Beta, Gamma, Delta and Omicron) and ELISA to quantify SARS-CoV-2 RBD-specific IgG subclasses. The results showed that the 2-dose primary immunization only achieved low neutralizing ability, while a booster shot can significantly enhance neutralizing ability against the wild-type strain, Beta, Gamma, Delta and Omicron variants. IgG1 and IgG3 were the most abundant serum antibodies, and IgG2 and IgG4 were hardly detected at any time. The ratio of IgG1/IgG3 was positively associated with the neutralization ability. The underlying mechanism requires further exploration.
Keywords: IgG subclasses; SARS-CoV-2 inactivated vaccine; neutralization ability; variants.
© 2022 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- UNICEF . COVID‐19 vaccine market dashboard (2022). Accessed 9 Feb 2022.
-
- Thomas JK. Vaccines in Kuby immunology. 6th ed. England: W. H. Freeman and Company; 2006. p. 484.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

