Immune checkpoint blockades in gynecological cancers: A review of clinical trials
- PMID: 35751489
- PMCID: PMC9564814
- DOI: 10.1111/aogs.14412
Immune checkpoint blockades in gynecological cancers: A review of clinical trials
Abstract
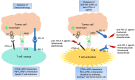
Advanced and recurrent gynecological cancers are associated with a poor prognosis and there is still a lack of effective treatments. Immune checkpoint blockade (ICB) therapy is an important element of cancer-targeted therapy and immunotherapy. The programed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) pathways are the two main targets of ICB. In this study, we provide a comprehensive review of clinical evidence concerning ICB therapy in gynecological cancers and discuss future implications. All clinical trials of ICB therapy in gynecological cancers were reviewed. We searched ClinicalTrials.gov to collect data from completed and ongoing clinical trials. The clinical evidence regarding the efficacy of ICB agents in gynecological cancers were discussed. Six phase III clinical trials have reported their results of primary outcomes, and a total of 25 phase II clinical trials have been completed. As revealed in phase III trials, pembrolizumab (a PD-1 antibody) improved the overall survival and progression-free survival in endometrial cancer patients with mismatch repair deficiency and cervical cancer patients with expressions of PD-L1. Based on these findings, pembrolizumab was approved by the Food and Drug Administration and European Medicines Agency as a cancer medication used to treat certain patients with endometrial cancer or cervical cancer. Other PD-1 antibodies, including dostarlimab and cemiplimab, also showed antitumor efficacy in clinical trials. Dostarlimab treatment showed an encouraging response rate in endometrial cancer patients with mismatch repair deficiency. Cemiplimab treatment led to a longer overall survival and a lower risk of death than chemotherapy among patients with recurrent cervical cancer. Three completed phase III trials investigated anti-PD-L1 agents (atezolizumab and avelumab) in the treatment of ovarian cancer. The results were not encouraging. Other strategies of ICB therapy which had showed potential clinical benefit in the treatment of gynecological cancers in early-phase trials need to be further evaluated in late-stage trials. The antitumor efficacy of ICB therapy is promising, and the key to making further progress in the treatment of gynecological cancers is to identify more biomarkers and explore innovative combination treatments with other targeted therapies.
Keywords: cervical cancer; clinical trials; endometrial cancer; immune checkpoint blockades; ovarian cancer.
© 2022 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures
Similar articles
-
PRIMMO study protocol: a phase II study combining PD-1 blockade, radiation and immunomodulation to tackle cervical and uterine cancer.BMC Cancer. 2019 May 28;19(1):506. doi: 10.1186/s12885-019-5676-3. BMC Cancer. 2019. PMID: 31138229 Free PMC article. Clinical Trial.
-
A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer.Am Soc Clin Oncol Educ Book. 2020 Mar;40:1-7. doi: 10.1200/EDBK_280503. Am Soc Clin Oncol Educ Book. 2020. PMID: 32213091 Review.
-
Optimizing immunotherapy for gynecologic cancers.Curr Opin Obstet Gynecol. 2020 Feb;32(1):1-8. doi: 10.1097/GCO.0000000000000603. Curr Opin Obstet Gynecol. 2020. PMID: 31833942 Free PMC article. Review.
-
The evolving role of immune checkpoint inhibitors in cervical and endometrial cancer.Cancer Drug Resist. 2024 Jun 11;7:23. doi: 10.20517/cdr.2023.120. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39050882 Free PMC article. Review.
-
Balstilimab and other immunotherapy for recurrent and metastatic cervical cancer.Med Oncol. 2022 Jan 29;39(4):47. doi: 10.1007/s12032-022-01646-7. Med Oncol. 2022. PMID: 35092506 Review.
Cited by
-
Tumour-infiltrating lymphocytes: from prognosis to treatment selection.Br J Cancer. 2023 Feb;128(3):451-458. doi: 10.1038/s41416-022-02119-4. Epub 2022 Dec 23. Br J Cancer. 2023. PMID: 36564565 Free PMC article.
-
Immune checkpoint inhibitors in gynecological cancers: a narrative review on the practice-changing trials.Immunotherapy. 2025 Jan;17(1):57-66. doi: 10.1080/1750743X.2025.2460964. Epub 2025 Feb 1. Immunotherapy. 2025. PMID: 39893504 Review.
-
Integrated machine learning identifies a cellular senescence-related prognostic model to improve outcomes in uterine corpus endometrial carcinoma.Front Immunol. 2024 Jun 27;15:1418508. doi: 10.3389/fimmu.2024.1418508. eCollection 2024. Front Immunol. 2024. PMID: 38994352 Free PMC article.
-
Hormone Receptors and Epithelial Ovarian Cancer: Recent Advances in Biology and Treatment Options.Biomedicines. 2023 Aug 1;11(8):2157. doi: 10.3390/biomedicines11082157. Biomedicines. 2023. PMID: 37626654 Free PMC article. Review.
-
Regional immune mechanisms enhance efficacy of an autologous cellular cancer vaccine with intraperitoneal administration.Oncoimmunology. 2024 Dec 31;13(1):2421029. doi: 10.1080/2162402X.2024.2421029. Epub 2024 Nov 1. Oncoimmunology. 2024. PMID: 39625271 Free PMC article.
References
-
- Zahn LM. Effects of the tumor microenvironment. Science. 2017;355:1386‐1388. - PubMed
-
- Neoadjuvant PD‐1 Blockade in Resectable Lung Cancer; Nivolumab and ipilimumab in advanced melanoma; overall survival with combined nivolumab and ipilimumab in advanced melanoma; prolonged survival in stage III melanoma with ipilimumab adjuvant therapy; combined nivolumab and ipilimumab or monotherapy in untreated melanoma; combined nivolumab and ipilimumab or monotherapy in untreated melanoma; nivolumab and ipilimumab versus ipilimumab in untreated melanoma; rapid eradication of a bulky melanoma mass with one dose of immunotherapy; genetic basis for clinical response to CTLA‐4 blockade; genetic basis for clinical response to CTLA‐4 blockade in melanoma; nivolumab plus ipilimumab in advanced melanoma; safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma; hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2018;379(22):2185. - PubMed
-
- Vogel I, Kasran A, Cremer J, et al. CD28/CTLA‐4/B7 costimulatory pathway blockade affects regulatory T‐cell function in autoimmunity. Eur J Immunol. 2015;45:1832‐1841. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials