Polygenic risk score for ACE-inhibitor-associated cough based on the discovery of new genetic loci
- PMID: 35751511
- PMCID: PMC10148738
- DOI: 10.1093/eurheartj/ehac322
Polygenic risk score for ACE-inhibitor-associated cough based on the discovery of new genetic loci
Abstract
Aims: To search for sequence variants associated with ACEi discontinuation and to test their association with ACEi-associated adverse drug reactions (ADRs).
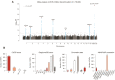
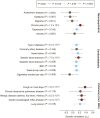
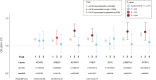
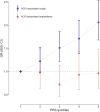
Methods and results: A genome-wide association study (GWAS) on ACEi discontinuation was conducted, including 33 959 ACEi-discontinuers and 44 041 controls. Cases were defined as persons who switched from an ACEi treatment to an angiotensin receptor blocker. Controls were defined as persons who continued ACEi treatment for at least 1 year. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were computed for ACEi discontinuation risk by mixed model regression analysis. Summary statistics from the individual cohorts were meta-analyzed with a fixed-effects model. To test for association with specific ACEi-associated ADRs, any genome-wide significant (P < 5 × 10-8) ACEi discontinuation variants was tested for association with ACEi-associated cough and angioedema. A polygenetic risk score (PRS) based on ACEi discontinuation GWAS data was constructed and tested for association with ACEi-associated cough and angioedema in two population-based samples. In total, seven genetic genome-wide loci were identified, of which six were previously unreported. The strongest association with ACEi discontinuation was at 20q13.3 (NTSR1; OR: 1.21; 95% CI: 1.17-1.24; P = 2.1 × 10-34). Five of seven lead variants were associated with ACEi-associated cough, whereas none were associated with ACEi-associated angioedema. The ACEi discontinuation PRS was associated with ACEi-associated cough in a dose-response manner but not with ACEi-associated angioedema. ACEi discontinuation was genetically correlated with important causes for cough, including gastro-esophageal reflux disease, allergic rhinitis, hay fever, and asthma, which indicates partly shared genetic underpinning between these traits.
Conclusion: This study showed the advantage of using prescription patterns to discover genetic links with ADRs. In total, seven genetic loci that associated with ACEi discontinuation were identified. There was evidence of a strong association between our ADR phenotype and ACEi-associated cough. Taken together, these findings increase insight into the pathophysiological processes that underlie ACEi-associated ADRs.
Keywords: ACE inhibitors; ACE-inhibitor associated cough; ADR; Adverse drug reaction; Drug discontinuation; GWAS; Genome-wide association study.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: The authors who are affiliated with deCODE genetics/Amgen Inc. declare competing financial interests as employees. C.T.P. reports grants from Bayer and grants from Novo Nordisk, outside the submitted work. L.K. reports personal fees and speakers honorarium for Novartis, AstraZeneca, and Boehringer, outside the submitted work. H.B. receives lecture fees from Bristol-Myers Squibb, Merch Sharp and Dohme. S.B. is a board member for Proscion A/S and Intomics A/S. All other authors declare that there is no conflict of interest.
Figures

Comment in
-
Toward personalized medicine for cardiovascular pharmacotherapy.Eur Heart J. 2022 Dec 1;43(45):4719-4721. doi: 10.1093/eurheartj/ehac413. Eur Heart J. 2022. PMID: 35924414 No abstract available.
Similar articles
-
Meta-analysis of ACE inhibitor-induced angioedema identifies novel risk locus.J Allergy Clin Immunol. 2024 Apr;153(4):1073-1082. doi: 10.1016/j.jaci.2023.11.921. Epub 2024 Jan 31. J Allergy Clin Immunol. 2024. PMID: 38300190
-
Documented Adverse Drug Reactions and Discontinuation of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Chronic Kidney Disease.Am J Nephrol. 2023;54(3-4):126-135. doi: 10.1159/000530988. Epub 2023 May 18. Am J Nephrol. 2023. PMID: 37231800 Free PMC article.
-
Exome Sequencing Reveals Common and Rare Variants in F5 Associated With ACE Inhibitor and Angiotensin Receptor Blocker-Induced Angioedema.Clin Pharmacol Ther. 2020 Dec;108(6):1195-1202. doi: 10.1002/cpt.1927. Epub 2020 Jul 18. Clin Pharmacol Ther. 2020. PMID: 32496628 Free PMC article.
-
Angiotensin converting enzyme inhibitors and the allergist.Ann Allergy. 1990 Jun;64(6):503-6. Ann Allergy. 1990. PMID: 2189318 Review.
-
Pharmacogenetics of angiotensin-converting enzyme inhibitor-induced angioedema.Clin Exp Allergy. 2019 Feb;49(2):142-154. doi: 10.1111/cea.13326. Epub 2019 Jan 8. Clin Exp Allergy. 2019. PMID: 30537422 Review.
Cited by
-
Association of SLCO1B1 gene variants with angiotensin-converting enzyme inhibitor-induced cough in a Pakistani hypertensive cohort.Front Pharmacol. 2024 Sep 4;15:1441251. doi: 10.3389/fphar.2024.1441251. eCollection 2024. Front Pharmacol. 2024. PMID: 39295941 Free PMC article.
-
Genome-wide associations spanning 194 in-hospital drug dosage change phenotypes highlight diverse genetic backgrounds in concurrent drug therapy.Comput Struct Biotechnol J. 2025 Jun 25;28:239-248. doi: 10.1016/j.csbj.2025.06.042. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40678446 Free PMC article.
-
Association between angiotensin-converting enzyme inhibitor-induced cough and the risk of lung cancer: a Mendelian randomization study.Front Pharmacol. 2023 Sep 20;14:1267924. doi: 10.3389/fphar.2023.1267924. eCollection 2023. Front Pharmacol. 2023. PMID: 37799968 Free PMC article.
-
High-Throughput Screening for Prescribing Cascades Among Real-World Angiotensin-Converting Enzyme Inhibitor Initiators.Pharmacoepidemiol Drug Saf. 2025 Mar;34(3):e70132. doi: 10.1002/pds.70132. Pharmacoepidemiol Drug Saf. 2025. PMID: 40098294
-
From genomic spectrum of NTRK genes to adverse effects of its inhibitors, a comprehensive genome-based and real-world pharmacovigilance analysis.Front Pharmacol. 2024 Jan 31;15:1329409. doi: 10.3389/fphar.2024.1329409. eCollection 2024. Front Pharmacol. 2024. PMID: 38357305 Free PMC article.
References
-
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. . 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevationThe Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. - PubMed
-
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. . 2018 ESC/ESH Guidelines for the management of arterial hypertensionThe Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 2018;39:3021–3104. - PubMed
-
- American Diabetes Association . 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care 2020;43(Suppl 1):S111–S134. - PubMed
-
- Verbeke F, Lindley E, Van Bortel L, Vanholder R, London G, Cochat P, et al. . A European renal best practice (ERBP) position statement on the kidney disease: improving global outcomes (KDIGO) clinical practice guideline for the management of blood pressure in non-dialysis-dependent chronic kidney disease: an endorsement with some caveats for real-life application. Nephrol Dial Transplant 2014;29:490–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- S10 RR025141/RR/NCRR NIH HHS/United States
- U01 HG004424/HG/NHGRI NIH HHS/United States
- U01 HG006389/HG/NHGRI NIH HHS/United States
- U01 HG006380/HG/NHGRI NIH HHS/United States
- R01 HD074711/HD/NICHD NIH HHS/United States
- RC2 GM092618/GM/NIGMS NIH HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- U01 HG006379/HG/NHGRI NIH HHS/United States
- S10 OD017985/OD/NIH HHS/United States
- U01 HG006385/HG/NHGRI NIH HHS/United States
- U01 HG006375/HG/NHGRI NIH HHS/United States
- U01 HG004438/HG/NHGRI NIH HHS/United States
- U01 HG006382/HG/NHGRI NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- U01 HG006388/HG/NHGRI NIH HHS/United States
- U01 HG006378/HG/NHGRI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- R01 NS032830/NS/NINDS NIH HHS/United States
- U01 HG004798/HG/NHGRI NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

