Modified-release nicotinamide for the treatment of hyperphosphataemia in haemodialysis patients: 52-week efficacy and safety results of the phase 3 randomized controlled NOPHOS trial
- PMID: 35751625
- PMCID: PMC10064978
- DOI: 10.1093/ndt/gfac206
Modified-release nicotinamide for the treatment of hyperphosphataemia in haemodialysis patients: 52-week efficacy and safety results of the phase 3 randomized controlled NOPHOS trial
Abstract
Background: We previously reported that modified-release nicotinamide (NAMR) was superior to placebo in reducing serum phosphate concentrations over 12 weeks in a large cohort of haemodialysis patients with hyperphosphataemia. Here we report outcomes after 52 weeks of treatment.
Methods: NOPHOS was a phase 3, international, randomized, controlled, double-blind trial with a parallel group design. NAMR (250-1500 mg/day) was investigated in comparison to placebo as an add-on therapy to an individual therapy with approved phosphate binders.
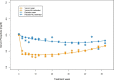
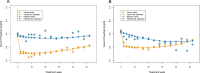
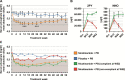
Results: In the intention-to-treat population (NAMR: n = 539; placebo: n = 183), serum phosphate was significantly lower in the NAMR group compared with the placebo group at week 24 (5.40 ± 1.55 versus 5.79 ± 1.37 mg/dl, P < .001) with a mean difference of -0.39 mg/dl [95% confidence interval (CI) -0.66 to -0.13], but was comparable between the groups at week 52 [mean difference -0.08 (95% CI -0.36-0.20)]. In the completer population (n = 358), statistical significance in favour of NAMR was reached at weeks 24 and 52. The treatment effect was reduced in patients with high baseline serum intact parathyroid hormone (iPTH) compared with patients with low baseline serum iPTH. Compliant patients in the NAMR group had a more pronounced and sustained reduction in serum phosphate than non-compliant patients. NAMR treatment was associated with a significantly increased risk of thrombocytopenia, pruritus, anaemia, and diarrhoea. Herpes zoster occurred exclusively in patients randomized to NAMR.
Conclusions: NAMR combined with phosphate binders significantly reduced serum phosphate over the first 24 weeks of treatment, but the treatment effect was not maintained up to week 52. Non-compliance may have contributed to reduced long-term efficacy. Several newly identified safety signals warrant further evaluation.
Keywords: haemodialysis; hyperphosphataemia; mineral and bone disease; nicotinamide; randomized controlled trial.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
M.Ke. has received lecture and consulting honoraria from Amgen, FMC, MEDICE, Sanofi, Vifor Fresenius Medical Care Renal Pharma and Vifor Pharma. A.W. has received honoraria for lectures from Fresenius, Bayer AG and MEDICE and for participation in Scientific Advisory Boards from GlaxoSmithKline. C.O. reports grants from MEDICE. A.R.R., J.R. and H.L. have no conflicts of interests to declare. B.H., M.Ka. and M.R. are employees of MEDICE Arzneimittel Pütter GmbH & Co. KG. R.A. is chief executive officer and owner of MEDICE Arzneimittel Pütter GmbH & Co. KG. The 52-week efficacy and safety results presented in this paper have not been published previously in whole or part. We previously reported the 12-week results from this trial in Ketteler M, Wiecek A, Rosenkranz A
Figures

References
-
- Block GA, Klassen PS, Lazarus JMet al. . Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208–18. - PubMed
-
- Kalantar-Zadeh K, Kuwae N, Regidor DLet al. . Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006;70:771–80. - PubMed
-
- Tentori F, Blayney MJ, Albert JMet al. . Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52:519–30. - PubMed
-
- Nakai S, Akiba T, Kazama Jet al. . Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial 2008;12:49–54. - PubMed

