Methionine oxidation activates pyruvate kinase M2 to promote pancreatic cancer metastasis
- PMID: 35752173
- PMCID: PMC9391305
- DOI: 10.1016/j.molcel.2022.06.005
Methionine oxidation activates pyruvate kinase M2 to promote pancreatic cancer metastasis
Abstract
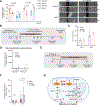
Cancer mortality is primarily a consequence of its metastatic spread. Here, we report that methionine sulfoxide reductase A (MSRA), which can reduce oxidized methionine residues, acts as a suppressor of pancreatic ductal adenocarcinoma (PDA) metastasis. MSRA expression is decreased in the metastatic tumors of PDA patients, whereas MSRA loss in primary PDA cells promotes migration and invasion. Chemoproteomic profiling of pancreatic organoids revealed that MSRA loss results in the selective oxidation of a methionine residue (M239) in pyruvate kinase M2 (PKM2). Moreover, M239 oxidation sustains PKM2 in an active tetrameric state to promote respiration, migration, and metastasis, whereas pharmacological activation of PKM2 increases cell migration and metastasis in vivo. These results demonstrate that methionine residues can act as reversible redox switches governing distinct signaling outcomes and that the MSRA-PKM2 axis serves as a regulatory nexus between redox biology and cancer metabolism to control tumor metastasis.
Keywords: PKM2; cancer metabolism; glucose oxidation; metastasis; methionine oxidation; pancreatic cancer; redox signaling.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.J.C., F.D.T., and A.H.C. are inventors on patent applications related to the redox-active reagents for methionine conjugation. C.J.T. is listed as an inventor on patents related to PKM2 activators. The remaining authors declare no competing interests.
Figures


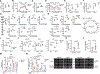


References
-
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, et al. (2011). Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283. 10.1126/science.1211485. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

