In vitro activity of a panel of per- and polyfluoroalkyl substances (PFAS), fatty acids, and pharmaceuticals in peroxisome proliferator-activated receptor (PPAR) alpha, PPAR gamma, and estrogen receptor assays
- PMID: 35752307
- PMCID: PMC9341220
- DOI: 10.1016/j.taap.2022.116136
In vitro activity of a panel of per- and polyfluoroalkyl substances (PFAS), fatty acids, and pharmaceuticals in peroxisome proliferator-activated receptor (PPAR) alpha, PPAR gamma, and estrogen receptor assays
Abstract
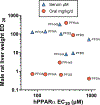
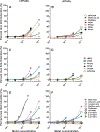
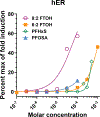
Data demonstrate numerous per- and polyfluoroalkyl substances (PFAS) activate peroxisome proliferator-activated receptor alpha (PPARα), however, additional work is needed to characterize PFAS activity on PPAR gamma (PPARγ) and other nuclear receptors. We utilized in vitro assays with either human or rat PPARα or PPARγ ligand binding domains to evaluate 16 PFAS (HFPO-DA, HFPO-DA-AS, NBP2, PFMOAA, PFHxA, PFOA, PFNA, PFDA, PFOS, PFBS, PFHxS, PFOSA, EtPFOSA, and 4:2, 6:2 and 8:2 FTOH), 3 endogenous fatty acids (oleic, linoleic, and octanoic), and 3 pharmaceuticals (WY14643, clofibrate, and the metabolite clofibric acid). We also tested chemicals for human estrogen receptor (hER) transcriptional activation. Nearly all compounds activated both PPARα and PPARγ in both human and rat ligand binding domain assays, except for the FTOH compounds and PFOSA. Receptor activation and relative potencies were evaluated based on effect concentration 20% (EC20), top percent of max fold induction (pmaxtop), and area under the curve (AUC). HFPO-DA and HFPO-DA-AS were the most potent (lowest EC20, highest pmaxtop and AUC) of all PFAS in rat and human PPARα assays, being slightly less potent than oleic and linoleic acid, while NBP2 was the most potent in rat and human PPARγ assays. Only PFHxS, 8:2 and 6:2 FTOH exhibited hER agonism >20% pmax. In vitro measures of human and rat PPARα and PPARγ activity did not correlate with oral doses or serum concentrations of PFAS that induced increases in male rat liver weight from the National Toxicology Program 28-d toxicity studies. Data indicate that both PPARα and PPARγ activation may be molecular initiating events that contribute to the in vivo effects observed for many PFAS.
Keywords: Adverse outcome pathway; Bioactivity; GenX; Mechanism of action; Molecular initiating event.
Copyright © 2022. Published by Elsevier Inc.
Figures

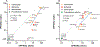
References
-
- Ashby J, Lefevre PA, Odum J, Tinwell H, Kennedy SJ, Beresford NA and Sumpter JP (1997). Failure to confirm estrogenic activity for benzoic acid and clofibrate: implications for lists of endocrine-disrupting agents. Regul Toxicol Pharmacol 26, 96–101. - PubMed
-
- ATSDR (2021). Toxicological Profile for Perfluoroalkyls. C5274127-A - PubMed
-
- Behr AC, Lichtenstein D, Braeuning A, Lampen A and Buhrke T (2018). Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol Lett 291, 51–60. - PubMed
-
- Behr AC, Plinsch C, Braeuning A and Buhrke T (2020). Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol In Vitro 62, 104700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

