ISM1 suppresses LPS-induced acute lung injury and post-injury lung fibrosis in mice
- PMID: 35752760
- PMCID: PMC9233842
- DOI: 10.1186/s10020-022-00500-w
ISM1 suppresses LPS-induced acute lung injury and post-injury lung fibrosis in mice
Abstract
Background: Acute lung injury/acute respiratory distress syndrome (ALI/ARDS) are clinical syndromes characterized by acute lung inflammation, pulmonary edema and hypoxemia, with up to 50% mortality rate without effective pharmacological therapy. Following the acute inflammation, repair and remodeling occurs which in some cases resulting in lung fibrosis. The pathophysiology of ALI/ARDS remains incompletely understood. Lipopolysaccharide (LPS)-induced ALI in mice have been widely used as a model to study human ALI/ARDS. Isthmin 1 (ISM1) is a secreted protein highly abundant in mouse lung. We have previously reported that upon intratracheal LPS instillation, ISM1 expression in the lung is further upregulated. Recently, we also reported that ISM1 is an anti-inflammatory protein in the lung with Ism1-/- mice presenting spontaneous chronic low-grade lung inflammation and obvious emphysema at young adult stage. However, what role ISM1 plays in ALI/ARDS and lung fibrosis remain unclear.
Methods: Using Ism1-/- mice and intratracheal LPS-induced ALI, and local delivery of recombinant ISM1 (rISM1), we investigated the role ISM1 plays in ALI and post-ALI lung fibrosis using flow cytometry, Western blot, antibody array, immunohistochemistry (IHC), immunofluorescent and other histological staining.
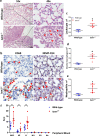
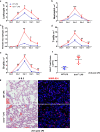
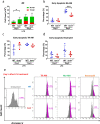
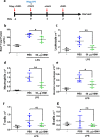
Results: We reveal that ISM1 deficiency in mice led to an intensified acute lung inflammation upon intratracheal LPS challenge, with a heightened leukocyte infiltration including neutrophils and monocyte-derived alveolar macrophages, as well as upregulation of multiple pro-inflammatory cytokines/chemokines including tumor necrosis factor α (TNF-α). Although innate immune cells largely subsided to the baseline by day 7 post-LPS challenge in both wild-type and Ism1-/- mice, Ism1-/- lung showed increased post-ALI fibrosis from day 9 post-LPS treatment with increased myofibroblasts, excessive collagen accumulation and TGF-β upregulation. The heightened lung fibrosis remained on day 28 post-LPS. Moreover, intranasal delivered recombinant ISM1 (rISM1) effectively suppressed LPS-induced acute lung inflammation and ALI, and rISM1 suppressed LPS-induced NF-κB activation in cultured mouse alveolar macrophages.
Conclusion: Together with our previous report, this work further established ISM1 as an endogenous anti-inflammation protein in the lung, restraining excessive host inflammatory response to LPS-triggered ALI and suppressing post-ALI lung fibrosis likely through suppressing NF-κB activation and pro-inflammatory cytokine/chemokine production.
Keywords: Acute lung injury; ISM1; Inflammation; LPS; Pulmonary fibrosis.
© 2022. The Author(s).
Conflict of interest statement
N.N, T.Y.W.L, W.S.F.W and R.G. are co-inventors of a pending patent application related to this work; R.G. is the scientific founder of NovoBreeze Therapeutics Co. Ltd. All other authors declare no conflict of interest.
Figures
Similar articles
-
Isthmin-1 attenuates allergic Asthma by stimulating adiponectin expression and alveolar macrophage efferocytosis in mice.Respir Res. 2023 Nov 6;24(1):269. doi: 10.1186/s12931-023-02569-1. Respir Res. 2023. PMID: 37932719 Free PMC article.
-
Sulfasalazine ameliorates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress and nuclear factor-kappaB pathways.Int J Biochem Cell Biol. 2024 Apr;169:106530. doi: 10.1016/j.biocel.2024.106530. Epub 2024 Jan 19. Int J Biochem Cell Biol. 2024. PMID: 38246263
-
Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-induced acute lung injury.Am J Physiol Lung Cell Mol Physiol. 2017 Feb 1;312(2):L231-L242. doi: 10.1152/ajplung.00389.2016. Epub 2016 Dec 2. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27913426
-
Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome.Acta Pharm Sin B. 2022 Feb;12(2):600-620. doi: 10.1016/j.apsb.2021.08.009. Epub 2021 Aug 12. Acta Pharm Sin B. 2022. PMID: 34401226 Free PMC article. Review.
-
Therapeutic Targeting of NF-κB in Acute Lung Injury: A Double-Edged Sword.Cells. 2022 Oct 21;11(20):3317. doi: 10.3390/cells11203317. Cells. 2022. PMID: 36291185 Free PMC article. Review.
Cited by
-
The Role of Adipokines in Inflammatory Mechanisms of Obesity.Int J Mol Sci. 2022 Nov 29;23(23):14982. doi: 10.3390/ijms232314982. Int J Mol Sci. 2022. PMID: 36499312 Free PMC article. Review.
-
Role of Ferroptosis in Regulating the Epithelial-Mesenchymal Transition in Pulmonary Fibrosis.Biomedicines. 2023 Jan 9;11(1):163. doi: 10.3390/biomedicines11010163. Biomedicines. 2023. PMID: 36672671 Free PMC article. Review.
-
Isthmin1 Upregulation in the Intestinal Microenvironment During Salmonella Typhimurium Infection: Identification and Characterization of Isthmin1-Producing Cells.Microorganisms. 2025 May 30;13(6):1280. doi: 10.3390/microorganisms13061280. Microorganisms. 2025. PMID: 40572169 Free PMC article.
-
Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: pathogenesis and therapeutic implications.Cell Commun Signal. 2024 Apr 25;22(1):241. doi: 10.1186/s12964-024-01620-y. Cell Commun Signal. 2024. PMID: 38664775 Free PMC article. Review.
-
Protective Effects of Pasireotide in LPS-Induced Acute Lung Injury.Pharmaceuticals (Basel). 2025 Jun 22;18(7):942. doi: 10.3390/ph18070942. Pharmaceuticals (Basel). 2025. PMID: 40732232 Free PMC article.
References
-
- Chan TK, et al. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J Allergy Clin Immunol. 2016;138(84–96):e81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

