Efficacy of RTS,S/AS01E malaria vaccine administered according to different full, fractional, and delayed third or early fourth dose regimens in children aged 5-17 months in Ghana and Kenya: an open-label, phase 2b, randomised controlled trial
- PMID: 35753316
- PMCID: PMC9420828
- DOI: 10.1016/S1473-3099(22)00273-0
Efficacy of RTS,S/AS01E malaria vaccine administered according to different full, fractional, and delayed third or early fourth dose regimens in children aged 5-17 months in Ghana and Kenya: an open-label, phase 2b, randomised controlled trial
Erratum in
-
Correction to Lancet Infect Dis 2022; 22: 1329-42.Lancet Infect Dis. 2022 Nov;22(11):e310. doi: 10.1016/S1473-3099(22)00610-7. Epub 2022 Sep 9. Lancet Infect Dis. 2022. PMID: 36096147 Free PMC article. No abstract available.
Abstract
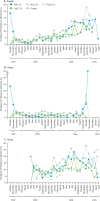
Background: Controlled infection studies in malaria-naive adults suggest increased vaccine efficacy for fractional-dose versus full-dose regimens of RTS,S/AS01. We report first results of an ongoing trial assessing different fractional-dose regimens in children, in natural exposure settings.
Methods: This open-label, phase 2b, randomised controlled trial is conducted at the Malaria Research Center, Agogo, Ashanti Region (Ghana), and the Kenya Medical Research Institute and the US Centers for Disease Control and Prevention site in Siaya County (Kenya). We enrolled children aged 5-17 months without serious acute or chronic illness who had previously received three doses of diphtheria, tetanus, pertussis, and hepatitis B vaccine and at least three doses of oral polio vaccine. Children were randomly assigned (1:1:1:1:1) using a web-based randomisation system with a minimisation procedure accounting for centre to receive rabies control vaccine (M012 schedule) or two full doses of RTS,S/AS01E at month 0 and month 1, followed by either full doses at months 2 and 20 (group R012-20 [standard regimen]), full doses at months 2, 14, 26, and 38 (R012-14), fractional doses at months 2, 14, 26, and 38 (Fx012-14), or fractional doses at months 7, 20, and 32 (Fx017-20). The fractional doses were administered as one fifth (0·1 mL) of the full RTS,S dose (0·5 mL) after reconstitution. All vaccines were administered by intramuscular injection in the left deltoid. The primary outcome was occurrence of clinical malaria cases from month 2·5 until month 14 for the Fx012-14 group versus the pooled R012-14 and R012-20 groups in the per-protocol set. We assessed incremental vaccine efficacy of the Fx012-14 group versus the pooled R012-14 and R012-20 group over 12 months after dose three. Safety was assessed in all children who received at least one vaccine dose. This trial is registered with ClinicalTrials.gov, NCT03276962.
Findings: Between Sept 28, 2017, and Sept 25, 2018, 2157 children were enrolled, of whom 1609 were randomly assigned to a treatment group (322 to each RTS,S/AS01E group and 321 to the rabies vaccine control group). 1500 children received at least one study vaccine dose and the per-protocol set comprised 1332 children. Over 12 months after dose three, the incremental vaccine efficacy in the Fx012-14 group versus the pooled R012-14 and R12-20 groups was -21% (95% CI -57 to 7; p=0·15). Up to month 21, serious adverse events occurred in 48 (16%) of 298 children in the R012-20 group, 45 (15%) of 294 in the R012-14 group, 47 (15%) of 304 in the Fx012-14 group, 62 (20%) of 311 in the Fx017-20 group, and 71 (24%) of 293 in the control group, with no safety signals observed.
Interpretation: The Fx012-14 regimen was not superior to the standard regimen over 12 months after dose three. All RTS,S/AS01E regimens provided substantial, similar protection against clinical malaria, suggesting potential flexibility in the recommended dosing regimen and schedule. This, and the effect of annual boosters, will be further evaluated through 50 months of follow-up.
Funding: GlaxoSmithKline Biologicals; PATH's Malaria Vaccine Initiative.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. OO-A, LS, ML, DM, ABo, FR, and EJ are employees of the GSK group of companies. OO-A, LS, DM, FR, and EJ have restricted shares in the GSK group of companies. All other authors declare no competing interests.
Figures





Comment in
-
Vaccination with fractional doses: promise or illusion?Lancet Infect Dis. 2022 Sep;22(9):1258-1259. doi: 10.1016/S1473-3099(22)00310-3. Epub 2022 Jun 23. Lancet Infect Dis. 2022. PMID: 35753319 No abstract available.
References
-
- WHO World malaria report 2021. http://www.who.int/publications/i/item/9789240040496
-
- Agnandji ST, Lell B, Soulanoudjingar SS, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. - PubMed
-
- WHO Malaria vaccine implementation programme (MVIP) 2020. http://www.who.int/news-room/q-a-detail/malaria-vaccine-implementation-p...
-
- The Lancet Malaria vaccine approval: a step change for global health. Lancet. 2021;398 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

