Somatostatin interneurons exhibit enhanced functional output and resilience to axotomy after mild traumatic brain injury
- PMID: 35753625
- PMCID: PMC9383472
- DOI: 10.1016/j.nbd.2022.105801
Somatostatin interneurons exhibit enhanced functional output and resilience to axotomy after mild traumatic brain injury
Abstract
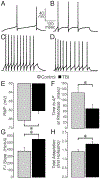
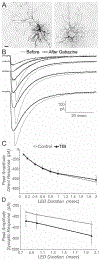
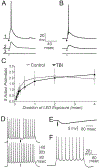
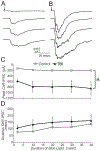
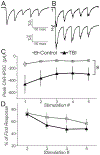
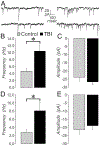
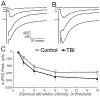
Mild traumatic brain injury (mTBI) gives rise to a remarkable breadth of pathobiological consequences, principal among which are traumatic axonal injury and perturbation of the functional integrity of neuronal networks that may arise secondary to the elimination of the presynaptic contribution of axotomized neurons. Because there exists a vast diversity of neocortical neuron subtypes, it is imperative to elucidate the relative vulnerability to axotomy among different subtypes. Toward this end, we exploited SOM-IRES-Cre mice to investigate the consequences of the central fluid percussion model of mTBI on the microanatomical integrity and the functional efficacy of the somatostatin (SOM) interneuron population, one of the principal subtypes of neocortical interneuron. We found that the SOM population is resilient to axotomy, representing only 10% of the global burden of inhibitory interneuron axotomy, a result congruous with past work demonstrating that parvalbumin (PV) interneurons bear most of the burden of interneuron axotomy. However, the intact structure of SOM interneurons after injury did not translate to normal cellular function. One day after mTBI, the SOM population is more intrinsically excitable and demonstrates enhanced synaptic efficacy upon post-synaptic layer 5 pyramidal neurons as measured by optogenetics, yet the global evoked inhibitory tone within layer 5 is stable. Simultaneously, there exists a significant increase in the frequency of miniature inhibitory post-synaptic currents within layer 5 pyramidal neurons. These results are consistent with a scheme in which 1 day after mTBI, SOM interneurons are stimulated to compensate for the release from inhibition of layer 5 pyramidal neurons secondary to the disproportionate axotomy of PV interneurons. The enhancement of SOM interneuron intrinsic excitability and synaptic efficacy may represent the initial phase of a dynamic process of attempted autoregulation of neocortical network homeostasis secondary to mTBI.
Keywords: Axonal injury; Cortical network; Inhibitory interneuron subtypes; Optogenetics; Traumatic brain injury.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures



References
-
- Bazarian JJ, et al., 2005. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj 19, 85–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

