Age-Limited Effects of Low-Frequency Transcutaneous Electric Nerve Stimulation on Insomnia: A 4-Week Multi-Center, Randomized Controlled Study
- PMID: 35753684
- PMCID: PMC9233949
- DOI: 10.30773/pi.2021.0374
Age-Limited Effects of Low-Frequency Transcutaneous Electric Nerve Stimulation on Insomnia: A 4-Week Multi-Center, Randomized Controlled Study
Abstract
Objective: Insomnia disorder is a common condition with considerable harmful effects on health. We investigated the therapeutic efficacy and safety of low-frequency transcutaneous electric nerve stimulation (LF-TENS) as an alternative treatment option for insomnia disorder.
Methods: A 4-week, multi-center, randomized controlled study was conducted. A total of 160 individuals aged 40 to 80 years with insomnia disorder were included and randomized to the experimental group receiving active device (n=81) or control group receiving sham device (n=79). Both groups used the device for four weeks, more than five days a week. The participants also completed pre- and post-intervention assessment with questionnaires, sleep diaries, wrist actigraphy, and blood tests.
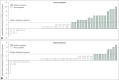
Results: There was no significant between-group difference in the changes of mood and sleep parameters and blood test results among the two study groups. Meanwhile, in the exploratory sub-group analysis of patients aged over 60 years, the experimental group showed better improvement after intervention in the change of Pittsburgh Sleep Quality Index (PSQI) score (-2.63±3.25 vs. -1.20±2.28, p=0.039; Cohen's d=0.99 vs. 0.45) and blood cortisol level (-1.65±3.37 μg/dL vs. -0.16±3.49 μg/dL, p=0.007; Cohen's d=0.56 vs. 0.05). In addition, no serious adverse reaction occurred during the study period in both groups.
Conclusion: The effect of LF-TENS was limited to older patients aged over 60 years, which might be related to the modulation of hypothalamic-pituitary-adrenal axis activity.
Keywords: Cortisol; Electrical stimulation; Insomnia; Sleep quality; Transcutaneous electric nerve stimulation.
Conflict of interest statement
Seockhoon Chung, a contributing editor of the
Figures

Similar articles
-
Effect of Transcranial Alternating Current Stimulation for the Treatment of Chronic Insomnia: A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Clinical Trial.Psychother Psychosom. 2020;89(1):38-47. doi: 10.1159/000504609. Epub 2019 Dec 17. Psychother Psychosom. 2020. PMID: 31846980 Clinical Trial.
-
Transcutaneous Vagus Nerve Stimulation for Insomnia in People Living in Places or Cities with High Altitudes: A Randomized Controlled Trial.Brain Sci. 2023 Jun 22;13(7):985. doi: 10.3390/brainsci13070985. Brain Sci. 2023. PMID: 37508917 Free PMC article.
-
Effects of trazodone versus cognitive behavioral therapy in the insomnia with short sleep duration phenotype: a preliminary study.J Clin Sleep Med. 2020 Dec 15;16(12):2009-2019. doi: 10.5664/jcsm.8740. J Clin Sleep Med. 2020. PMID: 32780015 Free PMC article. Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Placebo effect of acupuncture on insomnia: a systematic review and meta-analysis.Ann Palliat Med. 2020 Jan;9(1):19-29. doi: 10.21037/apm.2019.11.15. Ann Palliat Med. 2020. PMID: 32005059
References
-
- Lack L, Miller W, Turner D. A survey of sleeping difficulties in an Australian population. Community Health Stud. 1988;12:200–207. - PubMed
-
- National Institutes of Health National Institutes of Health State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep. 2005;28:1049–1057. - PubMed
-
- Morin CM, Bélanger L, LeBlanc M, Ivers H, Savard J, Espie CA, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–453. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

