Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review
- PMID: 35754071
- PMCID: PMC9349143
- DOI: 10.1007/s40262-022-01143-0
Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review
Abstract
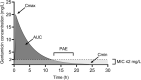
Gentamicin is an aminoglycoside antibiotic with a small therapeutic window that is currently used primarily as part of short-term empirical combination therapy. Gentamicin dosing schemes still need refinement, especially for subpopulations where pharmacokinetics can differ from pharmacokinetics in the general adult population: obese patients, critically ill patients, paediatric patients, neonates, elderly patients and patients on dialysis. This review summarizes the clinical pharmacokinetics of gentamicin in these patient populations and the consequences for optimal dosing of gentamicin for infections caused by Gram-negative bacteria, highlighting new insights from the last 10 years. In this period, several new population pharmacokinetic studies have focused on these subpopulations, providing insights into the typical values of the most relevant pharmacokinetic parameters, the variability of these parameters and possible explanations for this variability, although unexplained variability often remains high. Both dosing schemes and pharmacokinetic/pharmacodynamic (PK/PD) targets varied widely between these studies. A gentamicin starting dose of 7 mg/kg based on total body weight (or on adjusted body weight in obese patients) appears to be the optimal strategy for increasing the probability of target attainment (PTA) after the first administration for the most commonly used PK/PD targets in adults and children older than 1 month, including critically ill patients. However, evidence that increasing the PTA results in higher efficacy is lacking; no studies were identified that show a correlation between estimated or predicted PK/PD target attainment and clinical success. Although it is unclear if performing therapeutic drug monitoring (TDM) for optimization of the PTA is of clinical value, it is recommended in patients with highly variable pharmacokinetics, including patients from all subpopulations that are critically ill (such as elderly, children and neonates) and patients on intermittent haemodialysis. In addition, TDM for optimization of the dosing interval, targeting a trough concentration of at least < 2 mg/L but preferably < 0.5-1 mg/L, has proven to reduce nephrotoxicity and is therefore recommended in all patients receiving more than one dose of gentamicin. The usefulness of the daily area under the plasma concentration-time curve for predicting nephrotoxicity should be further investigated. Additionally, more research is needed on the optimal PK/PD targets for efficacy in the clinical situations in which gentamicin is currently used, that is, as monotherapy for urinary tract infections or as part of short-term combination therapy.
© 2022. The Author(s).
Conflict of interest statement
CH, AvdB, SdV, JP, RM and RvH declare that they have no conflict of interest.
Figures
References
-
- Matthews I, Kirkpatrick C, Holford N. Quantitative justification for target concentration intervention–parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol. 2004;58(1):8–19. doi: 10.1111/j.1365-2125.2004.02114.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

