Systematic measurements of interleaflet friction in supported bilayers
- PMID: 35754183
- PMCID: PMC9388387
- DOI: 10.1016/j.bpj.2022.06.023
Systematic measurements of interleaflet friction in supported bilayers
Abstract
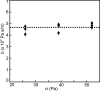
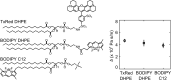
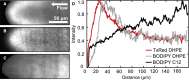
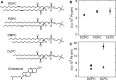
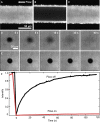
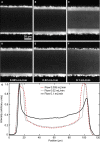
When lipid membranes curve or are subjected to strong shear forces, the two apposed leaflets of the bilayer slide past each other. The drag that one leaflet creates on the other is quantified by the coefficient of interleaflet friction, b. Existing measurements of this coefficient range over several orders of magnitude, so we used a recently developed microfluidic technique to measure it systematically in supported lipid membranes. Fluid shear stress was used to force the top leaflet of a supported membrane to slide over the stationary lower leaflet. Here, we show that this technique yields a reproducible measurement of the friction coefficient and is sensitive enough to detect differences in friction between membranes made from saturated and unsaturated lipids. Adding cholesterol to saturated and unsaturated membranes increased interleaflet friction significantly. We also discovered that fluid shear stress can reversibly induce gel phase in supported lipid bilayers that are close to the gel-transition temperature.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Keller S., Bezrukov S., et al. Parsegian V. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 1993;65:23–27. doi: 10.1016/s0006-3495(93)81040-3. https://linkinghub.elsevier.com/retrieve/pii/S0006349593810403 - DOI - PMC - PubMed
-
- Stone M.B., Shelby S.A., et al. Veatch S.L. Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. eLife. 2017;6:e19891. doi: 10.7554/elife.19891. https://elifesciences.org/articles/19891 - DOI - PMC - PubMed
-
- Raghupathy R., Anilkumar A.A., et al. Mayor S. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell. 2015;161:581–594. doi: 10.1016/j.cell.2015.03.048. https://linkinghub.elsevier.com/retrieve/pii/S0092867415003682 - DOI - PMC - PubMed
-
- Levental K.R., Malmberg E., et al. Levental I. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nat. Commun. 2020;11:1339. doi: 10.1038/s41467-020-15203-1. http://www.nature.com/articles/s41467-020-15203-1 - DOI - PMC - PubMed
-
- Falk K., Fillot N., et al. Loison C. Interleaflet sliding in lipidic bilayers under shear flow: comparison of the gel and fluid phases using reversed non-equilibrium molecular dynamics simulations. Phys. Chem. Chem. Phys. 2014;16:2154–2166. doi: 10.1039/c3cp53238k. http://xlink.rsc.org/?DOI=C3CP53238K - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

