Physiological and Pathophysiological Roles of Thioredoxin Interacting Protein: A Perspective on Redox Inflammation and Metabolism
- PMID: 35754346
- PMCID: PMC9968628
- DOI: 10.1089/ars.2022.0022
Physiological and Pathophysiological Roles of Thioredoxin Interacting Protein: A Perspective on Redox Inflammation and Metabolism
Abstract
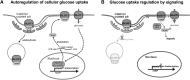
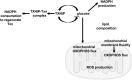
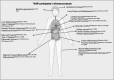
Significance: Thioredoxin interacting protein (TXNIP) is a member of the arrestin fold superfamily with important cellular functions, including cellular transport, mitochondrial energy generation, and protein cycling. It is the only arrestin-domain protein known to covalently bind to thioredoxin and plays roles in glucose metabolism, inflammation, apoptosis, and cancer. Recent Advances: The crystal structure of the TXNIP-thioredoxin complex provided details about this fascinating interaction. Recent studies showed that TXNIP is induced by endoplasmic reticulum (ER) stress, activates NLR family pyrin domain containing 3 (NLRP3) inflammasomes, and can regulate glucose transport into cells. The tumor suppressor role of TXNIP in various cancer types and the role of TXNIP in fructose absorption are now described. Critical Issues: The influence of TXNIP on redox state is more complex than its interaction with thioredoxin. Future Directions: It is incompletely understood which functions of TXNIP are thioredoxin-dependent. It is also unclear whether TXNIP binding can inhibit glucose transporters without endocytosis. TXNIP-regulated control of ER stress should also be investigated further. Antioxid. Redox Signal. 38, 442-460.
Keywords: TXNIP; arrestin; glucose; inflammasome; redox state; thioredoxin.
Conflict of interest statement
No competing financial interests exist.
Figures

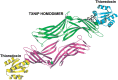

References
-
- Benovic JL, Kühn H, Weyand I, et al. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: Potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci U S A 1987;84(24):8879–8882; doi: 10.1073/pnas.84.24.8879 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

