Nanobodies as non-invasive imaging tools
- PMID: 35754459
- PMCID: PMC9216400
- DOI: 10.1016/j.iotech.2020.07.001
Nanobodies as non-invasive imaging tools
Abstract
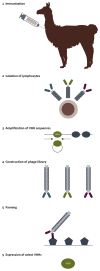
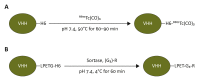
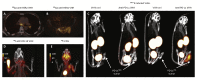
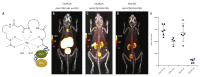
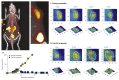
Antibodies and antibody fragments have found wide application for therapeutic and diagnostic purposes. Single-domain antibody fragments, also known as 'heavy-chain variable domains' or 'nanobodies', are a recent addition to the toolbox. Discovered some 30 years ago, nanobodies are the smallest antibody-derived fragments that retain antigen-binding properties. Their small size, stability, specificity, affinity and ease of manufacture make them appealing for use as imaging agents in the laboratory and the clinic. With the recent surge in immunotherapeutics and the success of cancer immunotherapy, it is important to be able to image immune responses and cancer biomarkers non-invasively to allocate resources and guide the best possible treatment of patients with cancer. This article reviews recent advances in the application of nanobodies as cancer imaging agents. While much work has been done in preclinical models, first-in-human applications are beginning to show the value of nanobodies as imaging agents.
Keywords: ImmunoPET; PET imaging; cancer biomarkers; immunotherapy; nanobody; non-invasive imaging.
© 2020 The Authors.
Figures
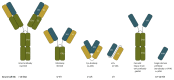
References
-
- Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. - PubMed
-
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. - PubMed
-
- Bensch F., van der Veen E.L., Hooge M.N.L., et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–1858. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

