Cytotoxicity of Amphotericin B and AmBisome: In Silico and In Vivo Evaluation Employing the Chick Embryo Model
- PMID: 35754489
- PMCID: PMC9214246
- DOI: 10.3389/fphar.2022.860598
Cytotoxicity of Amphotericin B and AmBisome: In Silico and In Vivo Evaluation Employing the Chick Embryo Model
Abstract
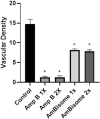
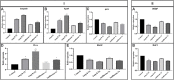
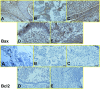
Leishmaniasis has been identified as a significant disease in tropical and subtropical regions of the world, with Iran being one of the disease-endemic areas. Various treatments have been applied for this disease, and amphotericin B (Amp B) is the second line of treatment. Side effects of this drug have been reported in various organs. The present study investigated the effects of different types of Amp B on fetal organs using in silico and in vivo assays (chicken embryos). In vivo analysis was done by checking pathological changes, angiogenesis, and apoptosis alterations on eggs treated by Amp B and AmBisome. In silico approach was employed to predict the affinity of Amp B and AmBisome to the vascular endothelial growth factor A (VEGF-A), its receptor (KDR1), apoptotic-regulator proteins (Bcl-2-associated X protein (Bax), B-cell lymphoma (Bcl-2), and Caspase-8. The ADME-toxicity prediction reveals that AmBisome possesses a superior pharmacological effect to Amp B. The best result of all the dockings in the Molegro Virtual Docker (MVD) was obtained between Bax, Bcl-2, Caspase-8, KDR1, and VEGF-A targets. Due to the lower Egap (HOMO-LUMO) of AmBisome, the chemical reactivity of AmBisome was higher than that of Amp B. In vivo analysis showed that embryos that received Amp B exhibited less vascular density than AmBisome. Amp B alone significantly increased the expression of apoptosis and decreased angiogenesis genes compared to AmBisome. The histopathology analysis of the treated embryos showed a reduction in the blood vessel collapse and an increase in degenerative and apoptotic-necrotic changes in the embryonic tissues. Overall, the results suggest the potential benefits of AmBisome over Amp B, which might be a better treatment strategy to treat leishmaniasis during pregnancy.
Keywords: amphotericin B; angiogenesis; apoptosis; chick embryo; in silico; in vivo; leishmaniasis; toxicity.
Copyright © 2022 Khosravi, Sharifi, Tavakkoli, Molaakbari, Bahraminegad, Salarkia, Seyedi, Keyhani, Salari, Sharifi, Bamorovat, Afgar and Dabiri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

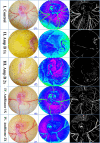


References
-
- Bahraminegad S., Pardakhty A., Sharifi I., Ranjbar M. (2021). The Assessment of Apoptosis, Toxicity Effects and Anti-leishmanial Study of Chitosan/CdO Core-Shell Nanoparticles, Eco-Friendly Synthesis and Evaluation. Arabian J. Chem. 14, 103085. 10.1016/j.arabjc.2021.103085 - DOI
-
- Bamorovat M., Sharifi I., Aflatoonian M. R., Sadeghi B., Shafiian A., Oliaee R. T., et al. (2019a). Host's Immune Response in Unresponsive and Responsive Patients with Anthroponotic Cutaneous Leishmaniasis Treated by Meglumine Antimoniate: A Case-Control Study of Th1 and Th2 Pathways. Int. Immunopharmacol. 69, 321–327. 10.1016/j.intimp.2019.02.008 - DOI - PubMed
-
- Bamorovat M., Sharifi I., Fekri A., Keyhani A., Aflatoonian M. R., Heshmatkhah A., et al. (2019b). A Single-Group Trial of End-Stage Patients with Anthroponotic Cutaneous Leishmaniasis: Levamisole in Combination with Glucantime in Field and Laboratory Models. Microb. Pathog. 128, 162–170. 10.1016/j.micpath.2018.12.040 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

