Occurrence of Strongylid Nematode Parasites on Horse Farms in Berlin and Brandenburg, Germany, With High Seroprevalence of Strongylus vulgaris Infection
- PMID: 35754549
- PMCID: PMC9226773
- DOI: 10.3389/fvets.2022.892920
Occurrence of Strongylid Nematode Parasites on Horse Farms in Berlin and Brandenburg, Germany, With High Seroprevalence of Strongylus vulgaris Infection
Abstract
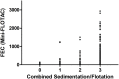
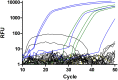
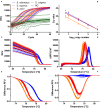
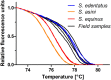
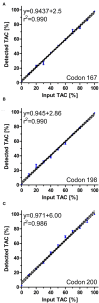
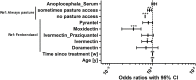
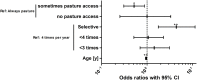
The infection of horses with strongylid nematodes is highly prevalent, with multi-species infections being the rule. Strongylus spp. and in particular Strongylus vulgaris are amongst the most pathogenic strongyle equine parasites. Presumably due to regular strategic anthelmintic treatments in combination with long prepatencies, prevalence of these worms was severely reduced in past decades. In this study, 484 horses from 48 farms in Berlin/Brandenburg, Germany were sampled between May 2017 and January 2018. Mini-FLOTAC and combined sedimentation/flotation were used to analyse faecal samples and larval cultures were carried out from individual strongyle infected horses for molecular testing for Strongylus spp. infection. Additionally, for Strongylus vulgaris, antibodies against a recombinant larval antigen were quantified in an ELISA. Strongyle type eggs were detected in 66.7% of the individual faecal samples. Nematode DNA was amplifiable from 311 samples and S. vulgaris and Strongylus edentatus were detected in four (1.3%) and 10 (6.3%) of these, respectively, the latter using a novel high-resolution-melt PCR targeting S. edentatus, Strongylus equinus, and Strongylus asini. On the farm level, prevalence for Strongylus spp. by PCR was 12.5%. Applying a conservative cut-off (sensitivity 0.43, specificity 0.96), 21.2% of all serum samples were positive for antibodies against S. vulgaris larvae (83.3% prevalence on farm level). Newly developed pyrosequencing assays to analyse putatively benzimidazole resistance associated polymorphisms in codons 167, 198, and 200 of the isotype 1 β-tubulin gene of S. vulgaris did not detect such polymorphisms in the four positive samples. Low age and increasing access to pasture were risk factors for egg shedding and seropositivity for S. vulgaris. Time since last treatment increased whereas use of moxidectin and ivermectin for the last treatment decreased the risk for strongyle egg shedding. Noteworthy, horses under selective treatment had significantly higher odds to be seropositive for anti-S. vulgaris antibodies than horses treated four times per year (odds ratio 4.4). The serological findings suggest that exposure to S. vulgaris is considerably higher than expected from direct diagnostic approaches. One potential explanation is the contamination of the environment by a few infected horses, leading to the infection of many horses with larvae that never reach maturity due to regular anthelmintic treatments.
Keywords: ELISA; Strongylus spp.; equine parasites; large strongyles; nematodes.
Copyright © 2022 Jürgenschellert, Krücken, Bousquet, Bartz, Heyer, Nielsen and von Samson-Himmelstjerna.
Conflict of interest statement
JB is employed by Virbac Tierarzneimittel GMBH and EB by Virbac France. GS-H declares that he has repeatedly acted as consultant for veterinary pharmaceutical and diagnostic companies and has previous and ongoing research collaborations with various companies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Comparison of morphological and molecular Strongylus spp. identification in equine larval cultures and first report of a patent Strongylus asini infection in a horse.Equine Vet J. 2025 Mar;57(2):522-529. doi: 10.1111/evj.14134. Epub 2024 Jul 16. Equine Vet J. 2025. PMID: 39012065 Free PMC article.
-
Prevalence of Strongylus vulgaris in horses after ten years of prescription usage of anthelmintics in Sweden.Vet Parasitol. 2019;276S:100013. doi: 10.1016/j.vpoa.2019.100013. Epub 2019 May 26. Vet Parasitol. 2019. PMID: 34311935
-
Prevalence of Strongylus vulgaris in horses after ten years of prescription usage of anthelmintics in Sweden.Vet Parasitol X. 2019 May 26;2:100013. doi: 10.1016/j.vpoa.2019.100013. eCollection 2019 Nov. Vet Parasitol X. 2019. PMID: 32904767 Free PMC article.
-
Detection and semi-quantification of Strongylus vulgaris DNA in equine faeces by real-time quantitative PCR.Int J Parasitol. 2008 Mar;38(3-4):443-53. doi: 10.1016/j.ijpara.2007.07.014. Epub 2007 Aug 14. Int J Parasitol. 2008. PMID: 17889881
-
The Strongylidae belonging to Strongylus genus in horses from southeastern Poland.Parasitol Res. 2012 Oct;111(4):1417-21. doi: 10.1007/s00436-012-3087-3. Epub 2012 Sep 8. Parasitol Res. 2012. PMID: 22961235 Free PMC article. Review.
Cited by
-
Presence of Equine and Bovine Coronaviruses, Endoparasites, and Bacteria in Fecal Samples of Horses with Colic.Pathogens. 2023 Aug 15;12(8):1043. doi: 10.3390/pathogens12081043. Pathogens. 2023. PMID: 37624003 Free PMC article.
-
A retrospective study of the prevalence in equine postmortems of cranial mesenteric arteritis caused by Strongylus vulgaris in Alberta (2010 to 2022).Can Vet J. 2024 Jun;65(6):587-593. Can Vet J. 2024. PMID: 38827589 Free PMC article.
-
Detection of SNPs and benzimidazole resistance in strongyle nematode eggs of horses by allele-specific PCR.Parasitol Res. 2023 Sep;122(9):2037-2043. doi: 10.1007/s00436-023-07903-6. Epub 2023 Jun 24. Parasitol Res. 2023. PMID: 37354256
-
Farm size and biosecurity measures associated with Strongylus vulgaris infection in horses.Equine Vet J. 2025 May;57(3):703-711. doi: 10.1111/evj.14212. Epub 2024 Aug 22. Equine Vet J. 2025. PMID: 39171858 Free PMC article.
-
Comparison of morphological and molecular Strongylus spp. identification in equine larval cultures and first report of a patent Strongylus asini infection in a horse.Equine Vet J. 2025 Mar;57(2):522-529. doi: 10.1111/evj.14134. Epub 2024 Jul 16. Equine Vet J. 2025. PMID: 39012065 Free PMC article.
References
-
- Kuzmina TA. Strongylids (nematoda: strongylidae) of domestic horses in Ukraine: modern state of Fauna and structure of the parasite community. Parazitologiia. (2012) 46:127–38. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

