Urease and α- Chymotrypsin Inhibitory Activities and Molecular Docking Studies of Alkaloids Isolated from Medicinal Plant Isatis minima Bunge
- PMID: 35754682
- PMCID: PMC9217576
- DOI: 10.1155/2022/1904874
Urease and α- Chymotrypsin Inhibitory Activities and Molecular Docking Studies of Alkaloids Isolated from Medicinal Plant Isatis minima Bunge
Abstract
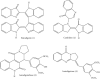
Phytochemical studies on the alkaloids fraction of the entire plant of Isatis minima Bunge resulted in the alkaloids 1-4 isolation, which were first time isolated from this species. The 1D and 2D NMR spectroscopic data were used to identify their structures, and there was satisfactory compatibility of the data compared to those which were previously published. In the examined compounds 1-4, Isaindigotidione (3) and Isaindigotone (4) were shown as an effective urease inhibitor in such a concentration-dependent way against Jack bean and Bacillus pasteurii urease, with IC50 values 29.03 ± 0.04, 20.04 ± 0.09 and 34.03 ± 0.07, 26.13 ± 0.08 μM, respectively. Compounds 3 and 4 were likewise shown to be an effective inhibitor against α-chymotrypsin, exhibiting IC50 values 16.09 ± 0.07 and 22.01 ± 0.06 μM, correspondingly. The program MOE-Dock was used to perform a molecular docking analysis to confirm probable binding modes of the active complexes of the isolated compounds 1-4 and the crystal structure of urease and α-chymotrypsin enzymes. Compound 3 was the most active, having the highest docking scores against Bacillus pasteurii urease, α-chymotrypsin, and Jack bean (-8.6876), (-7.6647), and (-13.1927) μM, respectively. All four alkaloids (1-4) showed significant urease and protease inhibitory potential and further these activities were confirmed with the help of molecular docking study.
Copyright © 2022 Fozia Fozia et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures




References
-
- Nasir Y. J., Ali S. I. Flora of Pakistan . Islamabad, Pakistan: National Herbarium Pakistan Agriculture Research Council; 1989.
-
- Zou P., Koh H. L. Determination of indican, isatin, indirubin and indigotin in Isatis indigotica by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up–to–the–Minute. Research in Mass Spectrometry . 2007;21(7):1239–1246. - PubMed
-
- Bourhia M., Amrati F. E. Z., Ullah R., et al. Coronavirus treatments: what drugs might work against COVID-19? Natural Product Communications . 2020;15(7) doi: 10.1177/1934578x20945442. - DOI
-
- Ministry of Public Health. Chinese Pharmacopoeia Beijing: Part-I . Bejing, China: Peoples Health Press; 1990.
LinkOut - more resources
Full Text Sources

