The type V myosin-containing complex HUM is a RAB11 effector powering movement of secretory vesicles
- PMID: 35754728
- PMCID: PMC9213775
- DOI: 10.1016/j.isci.2022.104514
The type V myosin-containing complex HUM is a RAB11 effector powering movement of secretory vesicles
Abstract
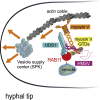
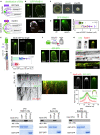
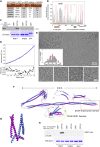
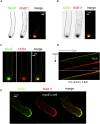
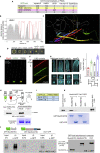
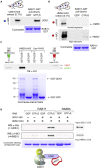
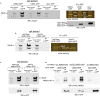
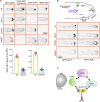
In the apex-directed RAB11 exocytic pathway of Aspergillus nidulans, kinesin-1/KinA conveys secretory vesicles (SVs) to the hyphal tip, where they are transferred to the type V myosin MyoE. MyoE concentrates SVs at an apical store located underneath the PM resembling the presynaptic active zone. A rod-shaped RAB11 effector, UDS1, and the intrinsically disordered and coiled-coil HMSV associate with MyoE in a stable HUM (HMSV-UDS1-MyoE) complex recruited by RAB11 to SVs through an interaction network involving RAB11 and HUM components, with the MyoE globular tail domain (GTD) binding both HMSV and RAB11-GTP and RAB11-GTP binding both the MyoE-GTD and UDS1. UDS1 bridges RAB11-GTP to HMSV, an avid interactor of the MyoE-GTD. The interaction between the UDS1-HMSV sub-complex and RAB11-GTP can be reconstituted in vitro. Ablating UDS1 or HMSV impairs actomyosin-mediated transport of SVs to the apex, resulting in spreading of RAB11 SVs across the apical dome as KinA/microtubule-dependent transport gains prominence.
Keywords: Biochemistry; Biological sciences; Molecular biology.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

