Coagulation factors promote brown adipose tissue dysfunction and abnormal systemic metabolism in obesity
- PMID: 35754738
- PMCID: PMC9218513
- DOI: 10.1016/j.isci.2022.104547
Coagulation factors promote brown adipose tissue dysfunction and abnormal systemic metabolism in obesity
Abstract
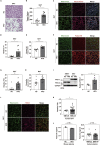
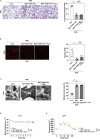
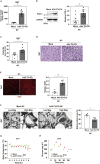
Brown adipose tissue (BAT) has a role in maintaining systemic metabolic health in rodents and humans. Here, we show that metabolic stress induces BAT to produce coagulation factors, which then-together with molecules derived from the circulation-promote BAT dysfunction and systemic glucose intolerance. When mice were fed a high-fat diet (HFD), the levels of tissue factor, coagulation Factor VII (FVII), activated coagulation Factor X (FXa), and protease-activated receptor 1 (PAR1) expression increased significantly in BAT. Genetic or pharmacological suppression of coagulation factor-PAR1 signaling in BAT ameliorated its whitening and improved thermogenic response and systemic glucose intolerance in mice with dietary obesity. Conversely, the activation of coagulation factor-PAR1 signaling in BAT caused mitochondrial dysfunction in brown adipocytes and systemic glucose intolerance in mice fed normal chow. These results indicate that BAT produces endogenous coagulation factors that mediate pleiotropic effects via PAR1 signaling under metabolic stress.
Keywords: Biological sciences; Cell biology; Human Physiology; Human metabolism.
© 2022 The Author(s).
Conflict of interest statement
The authors except for T.M. disclose no conflicts of interest. T.M. discloses the joint research funds and the remuneration for a lecture from Bayer; however, this company did not play a role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

