7-O-Galloyltricetifavan: a promising natural radical scavenger
- PMID: 35754988
- PMCID: PMC9214293
- DOI: 10.1098/rsos.211906
7-O-Galloyltricetifavan: a promising natural radical scavenger
Abstract
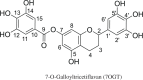
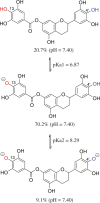
7-O-Galloyltricetifavan (7OGT), a natural flavonoid, is isolated from the leaves of Pithecellobium clypearia. The compound exhibits a variety of biological activities. This study details the evaluation of the HOO• antiradical activity of 7OGT by quantum chemistry calculations. The HOO• trapping activity of 7OGT in the gas phase (reference state) was discovered to follow the formal hydrogen transfer mechanism with a rate constant of k = 4.58 × 108 M-1 s-1. In physiological environments, 7OGT is predicted to be an excellent HOO• radical scavenger with k overall = 2.65 × 108 and 1.40 × 104 M-1 s-1 in water and pentyl ethanoate solvents, respectively. The HOO• antiradical activity of 7OGT in water at physiological pH is approximately 2000 times that of Trolox and substantially higher than that of other well-known natural antioxidants such as trans-resveratrol or ascorbic acid. Thus, 7OGT is an excellent natural antioxidant in polar environments.
Keywords: antioxidant; antiradical activity; density functional theory; flavonoid; kinetics.
© 2022 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
-
- Thao NP, Luyen BTT, Kim JH, Jo AR, Dat NT, Van Kiem P, Van Minh C, Kim YH. 2016. Identification, characterization, kinetics, and molecular docking of flavonoid constituents from Archidendron clypearia (Jack.) Nielsen leaves and twigs. Bioorg. Med. Chem. 24, 3125-3132. (10.1016/j.bmc.2016.05.034) - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources