Identifying Possible AChE Inhibitors from Drug-like Molecules via Machine Learning and Experimental Studies
- PMID: 35755364
- PMCID: PMC9219098
- DOI: 10.1021/acsomega.2c00908
Identifying Possible AChE Inhibitors from Drug-like Molecules via Machine Learning and Experimental Studies
Abstract
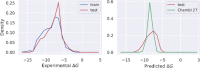
Acetylcholinesterase (AChE) is one of the most important drug targets for Alzheimer's disease (AD) treatment. In this work, a machine learning model was trained to rapidly and accurately screen large chemical databases for the potential inhibitors of AChE. The obtained results were then validated via in vitro enzyme assay. Moreover, atomistic simulations including molecular docking and molecular dynamics simulations were then used to understand molecular insights into the binding process of ligands to AChE. In particular, two compounds including benzyl trifluoromethyl ketone and trifluoromethylstyryl ketone were indicated as highly potent inhibitors of AChE because they established IC50 values of 0.51 and 0.33 μM, respectively. The obtained IC50 of two compounds is significantly lower than that of galantamine (2.10 μM). The predicted log(BB) suggests that the compounds may be able to traverse the blood-brain barrier. A good agreement between computational and experimental studies was observed, indicating that the hybrid approach can enhance AD therapy.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 2018, 14, 367. 10.1016/j.jalz.2018.02.001. - DOI
-
- Nasica-Labouze J.; Nguyen P. H.; Sterpone F.; Berthoumieu O.; Buchete N.-V.; Coté S.; De Simone A.; Doig A. J.; Faller P.; Garcia A.; Laio A.; Li M. S.; Melchionna S.; Mousseau N.; Mu Y.; Paravastu A.; Pasquali S.; Rosenman D. J.; Strodel B.; Tarus B.; Viles J. H.; Zhang T.; Wang C.; Derreumaux P. Amyloid β Protein and Alzheimer’s Disease: When Computer Simulations Complement Experimental Studies. Chem. Rev. 2015, 115, 3518–3563. 10.1021/cr500638n. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

