Multivalent Interaction of Beta-Catenin With its Intrinsically Disordered Binding Partner Adenomatous Polyposis Coli
- PMID: 35755812
- PMCID: PMC9214244
- DOI: 10.3389/fmolb.2022.896493
Multivalent Interaction of Beta-Catenin With its Intrinsically Disordered Binding Partner Adenomatous Polyposis Coli
Abstract
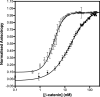
The Wnt signalling pathway plays key roles in cell proliferation, differentiation and fate decisions in embryonic development and maintenance of adult tissues, and the twelve Armadillo (ARM) repeat-containing protein β-catenin acts as the signal transducer in this pathway. Here we investigate the interaction between β-catenin's ARM repeat domain and the intrinsically disordered protein adenomatous polyposis coli (APC). APC is a giant multivalent scaffold that brings together the different components of the so-called "β-catenin destruction complex", which drives β-catenin degradation via the ubiquitin-proteasome pathway. Mutations and truncations in APC, resulting in loss of APC function and hence elevated β-catenin levels and upregulation of Wnt signalling, are associated with numerous cancers including colorectal carcinomas. APC has a long intrinsically disordered region (IDR) that contains a series of 15-residue and 20-residue binding regions for β-catenin. Here we explore the multivalent nature of the interaction of β-catenin with the highest affinity APC repeat, both at equilibrium and under kinetic conditions. We use a combination of single-site substitutions, deletions and insertions to dissect the mechanism of molecular recognition and the roles of the three β-catenin-binding subdomains of APC.
Keywords: adenomatous polyposis coli (APC); armadillo repeat; beta-catenin (β-catenin); fuzzy binding; intrinsically disordered protein; multivalency; protein-protein interaction (PPI).
Copyright © 2022 Rowling, Murton, Du and Itzhaki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Parallel and Sequential Pathways of Molecular Recognition of a Tandem-Repeat Protein and Its Intrinsically Disordered Binding Partner.Biomolecules. 2021 Jun 1;11(6):827. doi: 10.3390/biom11060827. Biomolecules. 2021. PMID: 34206070 Free PMC article.
-
The 15-Amino Acid Repeat Region of Adenomatous Polyposis Coli Is Intrinsically Disordered and Retains Conformational Flexibility upon Binding β-Catenin.Biochemistry. 2020 Oct 20;59(41):4039-4050. doi: 10.1021/acs.biochem.0c00479. Epub 2020 Oct 1. Biochemistry. 2020. PMID: 32941008 Free PMC article.
-
Adenomatous polyposis coli (APC) membrane recruitment 3, a member of the APC membrane recruitment family of APC-binding proteins, is a positive regulator of Wnt-β-catenin signalling.FEBS J. 2014 Feb;281(3):787-801. doi: 10.1111/febs.12624. Epub 2013 Dec 12. FEBS J. 2014. PMID: 24251807
-
A perspective on medicinal chemistry approaches towards adenomatous polyposis coli and Wnt signal based colorectal cancer inhibitors.Eur J Med Chem. 2021 Feb 15;212:113149. doi: 10.1016/j.ejmech.2020.113149. Epub 2021 Jan 3. Eur J Med Chem. 2021. PMID: 33445154 Review.
-
The Yin-Yang of TCF/beta-catenin signaling.Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review.
Cited by
-
Mechanism of APC truncation involved in colorectal cancer tumorigenesis (Review).Oncol Lett. 2024 Oct 15;29(1):2. doi: 10.3892/ol.2024.14748. eCollection 2025 Jan. Oncol Lett. 2024. PMID: 39526304 Free PMC article. Review.
-
Glycogen synthase kinase 3 inhibition controls Mycobacterium tuberculosis infection.iScience. 2024 Jul 20;27(8):110555. doi: 10.1016/j.isci.2024.110555. eCollection 2024 Aug 16. iScience. 2024. PMID: 39175770 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

