New β-Propellers Are Continuously Amplified From Single Blades in all Major Lineages of the β-Propeller Superfamily
- PMID: 35755816
- PMCID: PMC9218822
- DOI: 10.3389/fmolb.2022.895496
New β-Propellers Are Continuously Amplified From Single Blades in all Major Lineages of the β-Propeller Superfamily
Abstract
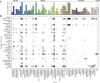
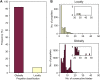
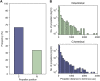
β-Propellers are toroidal folds, in which consecutive supersecondary structure units of four anti-parallel β-strands-called blades-are arranged radially around a central axis. Uniquely among toroidal folds, blades span the full range of sequence symmetry, from near identity to complete divergence, indicating an ongoing process of amplification and differentiation. We have proposed that the major lineages of β-propellers arose through this mechanism and that therefore their last common ancestor was a single blade, not a fully formed β-propeller. Here we show that this process of amplification and differentiation is also widespread within individual lineages, yielding β-propellers with blades of more than 60% pairwise sequence identity in most major β-propeller families. In some cases, the blades are nearly identical, indicating a very recent amplification event, but even in cases where such recently amplified β-propellers have more than 80% overall sequence identity to each other, comparison of their DNA sequence shows that the amplification occurred independently.
Keywords: amplification; differentiation; protein evolution; repetition; β-propeller.
Copyright © 2022 Pereira and Lupas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




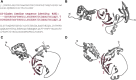

Similar articles
-
β-Propeller blades as ancestral peptides in protein evolution.PLoS One. 2013 Oct 15;8(10):e77074. doi: 10.1371/journal.pone.0077074. eCollection 2013. PLoS One. 2013. PMID: 24143202 Free PMC article.
-
Structural diversity of oligomeric β-propellers with different numbers of identical blades.Elife. 2019 Oct 15;8:e49853. doi: 10.7554/eLife.49853. Elife. 2019. PMID: 31613220 Free PMC article.
-
Sequence and structural analysis of the Asp-box motif and Asp-box beta-propellers; a widespread propeller-type characteristic of the Vps10 domain family and several glycoside hydrolase families.BMC Struct Biol. 2009 Jul 13;9:46. doi: 10.1186/1472-6807-9-46. BMC Struct Biol. 2009. PMID: 19594936 Free PMC article.
-
The many blades of the β-propeller proteins: conserved but versatile.Trends Biochem Sci. 2011 Oct;36(10):553-61. doi: 10.1016/j.tibs.2011.07.004. Epub 2011 Sep 15. Trends Biochem Sci. 2011. PMID: 21924917 Review.
-
Beta-propellers: associated functions and their role in human diseases.Curr Med Chem. 2003 Mar;10(6):505-24. doi: 10.2174/0929867033368204. Curr Med Chem. 2003. PMID: 12570695 Review.
Cited by
-
Piecing Together the History of Protein Folds From a Fragmented Evolutionary Record.Genome Biol Evol. 2025 Jul 30;17(8):evaf148. doi: 10.1093/gbe/evaf148. Genome Biol Evol. 2025. PMID: 40839423 Free PMC article.
-
Comparative analysis of thermal adaptations of extremophilic prolyl oligopeptidases.Biophys J. 2024 Sep 17;123(18):3143-3162. doi: 10.1016/j.bpj.2024.07.013. Epub 2024 Jul 15. Biophys J. 2024. PMID: 39014897 Free PMC article.
-
Competition between inside-out unfolding and pathogenic aggregation in an amyloid-forming β-propeller.Nat Commun. 2024 Jan 2;15(1):155. doi: 10.1038/s41467-023-44479-2. Nat Commun. 2024. PMID: 38168102 Free PMC article.
References
LinkOut - more resources
Full Text Sources

