Limited Impacts of Cover Cropping on Soil N-Cycling Microbial Communities of Long-Term Corn Monocultures
- PMID: 35755999
- PMCID: PMC9226624
- DOI: 10.3389/fmicb.2022.926592
Limited Impacts of Cover Cropping on Soil N-Cycling Microbial Communities of Long-Term Corn Monocultures
Abstract
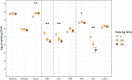
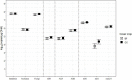
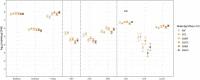
Cover cropping (CC) is a promising in-field practice to mitigate soil health degradation and nitrogen (N) losses from excessive N fertilization. Soil N-cycling microbial communities are the fundamental drivers of these processes, but how they respond to CC under field conditions is poorly documented for typical agricultural systems. Our objective was to investigate this relationship for a long-term (36 years) corn [Zea mays L.] monocultures under three N fertilizer rates (N0, N202, and N269; kg N/ha), where a mixture of cereal rye [Secale cereale L.] and hairy vetch [Vicia villosa Roth.] was introduced for two consecutive years, using winter fallows as controls (BF). A 3 × 2 split-plot arrangement of N rates and CC treatments in a randomized complete block design with three replications was deployed. Soil chemical and physical properties and potential nitrification (PNR) and denitrification (PDR) rates were measured along with functional genes, including nifH, archaeal and bacterial amoA, nirK, nirS, and nosZ-I, sequenced in Illumina MiSeq system and quantified in high-throughput quantitative polymerase chain reaction (qPCR). The abundances of nifH, archaeal amoA, and nirS decreased with N fertilization (by 7.9, 4.8, and 38.9 times, respectively), and correlated positively with soil pH. Bacterial amoA increased by 2.4 times with CC within N269 and correlated positively with soil nitrate. CC increased the abundance of nirK by 1.5 times when fertilized. For both bacterial amoA and nirK, N202 and N269 did not differ from N0 within BF. Treatments had no significant effects on nosZ-I. The reported changes did not translate into differences in functionality as PNR and PDR did not respond to treatments. These results suggested that N fertilization disrupts the soil N-cycling communities of this system primarily through soil acidification and high nutrient availability. Two years of CC may not be enough to change the N-cycling communities that adapted to decades of disruption from N fertilization in corn monoculture. This is valuable primary information to understand the potentials and limitations of CC when introduced into long-term agricultural systems.
Keywords: N cycle genes; N fertilization; amoA; maize (Zea mays L.); nifH; nirK; nirS; nosZ.
Copyright © 2022 Kim, Riggins, Zabaloy, Rodriguez-Zas and Villamil.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Acuña J. C. M., Villamil M. B. (2014). Short-term effects of cover crops and compaction on soil properties and soybean production in Illinois. Agron. J. 106 860–870. 10.2134/agronj13.0370 - DOI
-
- Bakari R., Mungai N., Thuita M., Masso C. (2020). Impact of soil acidity and liming on soybean (Glycine max) nodulation and nitrogen fixation in Kenyan soils. Acta Agric. Scand. B Soil Plant Sci. 70 667–678. 10.1080/09064710.2020.1833976 - DOI
-
- Barak P., Jobe B. O., Krueger A. R., Peterson L. A., Laird D. A. (1997). Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil. 197 61–69. 10.1023/A:1004297607070 - DOI
-
- Basche A. D., Miguez F. E., Kaspar T. C., Castellano M. J. (2014). Do cover crops increase or decrease nitrous oxide emissions? A meta-analysis. J. Soil Water Conserv. 69 471–482. 10.2489/jswc.69.6.471 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

