Comparison of Intestinal Microbes in Noninfectious Anterior Scleritis Patients With and Without Rheumatoid Arthritis
- PMID: 35756002
- PMCID: PMC9218904
- DOI: 10.3389/fmicb.2022.925929
Comparison of Intestinal Microbes in Noninfectious Anterior Scleritis Patients With and Without Rheumatoid Arthritis
Abstract
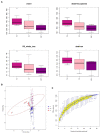
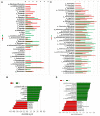
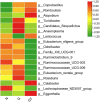
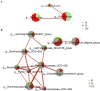
We compared intestinal microbes in anterior noninfectious scleritis patients with and without rheumatoid arthritis. Active noninfectious anterior scleritis patients without other immune diseases (G group, 16 patients) or with active rheumatoid arthritis (GY group, seven patients) were included in this study. Eight age- and sex-matched healthy subjects served as controls (N group). DNA was extracted from fecal samples. The V3-V4 16S rDNA region was amplified and sequenced by high-throughput 16S rDNA analysis, and microbial contents were determined. A significant decrease in species richness in the GY group was revealed by α- and β-diversity analyses (p = 0.02 and p = 0.004, respectively). At the genus level, 14 enriched and 10 decreased microbes in the G group and 13 enriched and 18 decreased microbes in the GY group were identified. Among them, four microbes were enriched in both the G and GY groups, including Turicibacter, Romboutsia, Atopobium, and Coprobacillus. Although two microbes (Lachnospiraceae_ND3007_group and Eggerthella) exhibited similar tendencies in the G and GY groups, changes in these microbes were more significant in the GY group (p < 0.05). Interaction analysis showed that Intestinibacter, Romboutsia, and Turicibacter, which were enriched in both the G and GY groups, correlated positively with each other. In addition, nine microbes were decreased in the GY group, which demonstrates a potential protective role for these microbes in the pathogenesis of scleritis via interactions with each other.
Keywords: Atopobium; Coprobacillus; Romboutsia; Turicibacter; intestinal microbes; noninfectious anterior scleritis; rheumatoid arthritis; scleritis.
Copyright © 2022 Li, Yang, Zhao, Bai and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O., 3rd, et al. (2010). 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588. doi: 10.1136/ard.2010.138461, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

